Design and synthesis of biologically active peptides: a 'tail' of amino acids can modulate activity of synthetic cyclic peptides
- PMID: 22015269
- PMCID: PMC3230782
- DOI: 10.1016/j.peptides.2011.10.007
Design and synthesis of biologically active peptides: a 'tail' of amino acids can modulate activity of synthetic cyclic peptides
Abstract
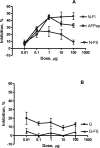
In earlier work, we synthesized a cyclic 9-amino acid peptide (AFPep, cyclo[EKTOVNOGN]) and showed it to be useful for prevention and therapy of breast cancer. In an effort to explore the structure-function relationships of AFPep, we have designed analogs that bear a short 'tail' (one or two amino acids) attached to the cyclic peptide distal to its pharmacophore. Analogs that bore a tail of either one or two amino acids, either of which had a hydrophilic moiety in the side chain (e.g., cyclo[EKTOVNOGN]FS) exhibited greatly diminished biological activity (inhibition of estrogen-stimulated uterine growth) relative to AFPep. Analogs that bore a tail of either one or two amino acids which had hydrophobic (aliphatic or aromatic) side chains (e.g., cyclo[EKTOVNOGN]FI) retained (or had enhanced) growth inhibition activity. Combining in the same biological assay a hydrophilic-tailed analog with either AFPep or a hydrophobic-tailed analog resulted in decreased activity relative to that for AFPep or for the hydrophobic-tailed analog alone, suggesting that hydrophilic-tailed analogs are binding to a biologically active receptor. An analog with a disrupted pharmacophore (cyclo[EKTOVGOGN]) exhibited little or no growth inhibition activity. An analog with a hydrophilic tail and a disrupted pharmacophore (cyclo[EKTOVGOGN]FS) exhibited no growth inhibition activity of its own and did not affect the activity of a hydrophobic-tailed analog, but enhanced the growth inhibition activity of AFPep. These results are discussed in the context of a two-receptor model for binding of AFPep and ring-and-tail analogs. We suggest that tails on cyclic peptides may comprise a useful method to enhance diversity of peptide design and specificity of ligand-receptor interactions.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Bennett JA, Defreest L, Anaka I, Saadati H, Balulad S, Jacobson HI, et al. AFPep: an anti-breast cancer peptide that is orally active. Breast Cancer Res Treat. 2006 Mar 15;98(2):133–41. - PubMed
-
- Bennett JA, Semeniuk DJ, Jacobson HI, Murgita RA. Similarity between natural and recombinant human alpha-fetoprotein as inhibitors of estrogen-dependent breast cancer growth. Breast Cancer Res Treat. 1997 Sep;45(2):169–79. - PubMed
-
- Bennett JA, Zhu S, Pagano-Mirarchi A, Kellom TA, Jacobson HI. Alpha-fetoprotein derived from a human hepatoma prevents growth of estrogen-dependent human breast cancer xenografts. Clin Cancer Res. 1998 Nov;4(11):2877–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

