The St. Gallen Prize Lecture 2011: evolution of long-term adjuvant anti-hormone therapy: consequences and opportunities
- PMID: 22015273
- PMCID: PMC3521565
- DOI: 10.1016/S0960-9776(11)70287-9
The St. Gallen Prize Lecture 2011: evolution of long-term adjuvant anti-hormone therapy: consequences and opportunities
Abstract
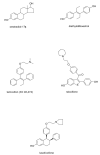
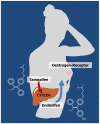
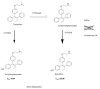
The successful translation of the scientific principles of targeting the breast tumour oestrogen receptor (ER) with the nonsteroidal anti-oestrogen tamoxifen and using extended durations (at least 5 years) of adjuvant therapy, dramatically increased patient survivorship and significantly enhanced a drop in national mortality rates from breast cancer. The principles are the same for the validation of aromatase inhibitors to treat post-menopausal patients but tamoxifen remains a cheap, life-saving medicine for the pre-menopausal patient. Results from the Oxford Overview Analysis illustrate the scientific principle of "longer is better" for adjuvant therapy in pre-menopausal patients. One year of adjuvant therapy is ineffective at preventing disease recurrence or reducing mortality, whereas five years of adjuvant tamoxifen reduces recurrence by 50% which is maintained for a further ten years after treatment stops. Mortality is reduced but the magnitude continues to increase to 30% over a 15-year period. With this clinical database, it is now possible to implement simple solutions to enhance survivorship. Compliance with long-term anti-hormone adjuvant therapy is critical. In this regard, the use of selective serotonin reuptake inhibitors (SSRIs) to reduce severe menopausal side effects may be inappropriate. It is known that SSRIs block the CYP2D6 enzyme that metabolically activates tamoxifen to its potent anti-oestrogenic metabolite, endoxifen. The selective norepinephrine reuptake inhibitor, venlafaxine, does not block CYP2D6, and may be a better choice. Nevertheless, even with perfect compliance, the relentless drive of the breast cancer cell to acquire resistance to therapy persists. The clinical application of long-term anti-hormonal therapy for the early treatment and prevention of breast cancer, focused laboratory research on the discovery of mechanisms involved in acquired anti-hormone resistance. Decades of laboratory study to reproduce clinical experience described not only the unique mechanism of selective ER modulator (SERM)-stimulated breast cancer growth, but also a new apoptotic biology of oestradiol action in breast cancer, following 5 years of anti-hormonal treatment. Oestradiol-induced apoptotic therapy is currently shown to be successful for the short-term treatment of metastatic ER positive breast cancer following exhaustive treatment with anti-hormones. The "oestrogen purge" concept is now being integrated into trials of long-term adjuvant anti-hormone therapy. The Study of Letrazole Extension (SOLE) trial employs "anti-hormonal drug holidays" so that a woman's own oestrogen may periodically purge and kill the nascent sensitized breast cancer cells that are developing. This is the translation of an idea first proposed at the 1992 St. Gallen Conference. Although tamoxifen is the first successful targeted therapy in cancer, the pioneering medicine is more than that. A study of the pharmacology of tamoxifen opened the door for a pioneering application in cancer chemoprevention and created a new drug group: the SERMs, with group members (raloxifene and lasofoxifene) approved for the treatment and prevention of osteoporosis with a simultaneous reduction of breast cancer risk. Thus, the combined strategies of long-term anti-hormone adjuvant therapy, targeted to the breast tumour ER, coupled with the expanding use of SERMs to prevent osteoporosis and prevent breast cancer as a beneficial side effect, have advanced patient survivorship significantly and promise to reduce breast cancer incidence.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



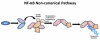


References
-
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–13. - PubMed
-
- Jordan VC. Selective estrogen receptor modulation: a personal perspective. Cancer Res. 2001;61:5683–7. - PubMed
-
- Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26:3073–82. - PubMed
-
- EBCTCG Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

