Cytoskeleton disruption in J774 macrophages: consequences for lipid droplet formation and cholesterol flux
- PMID: 22015387
- PMCID: PMC3274585
- DOI: 10.1016/j.bbalip.2011.09.015
Cytoskeleton disruption in J774 macrophages: consequences for lipid droplet formation and cholesterol flux
Abstract
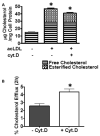
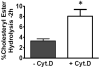
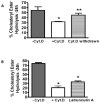
Macrophages store excess unesterified cholesterol (free, FC) in the form of cholesteryl ester (CE) in cytoplasmic lipid droplets. The hydrolysis of droplet-CE in peripheral foam cells is critical to HDL-promoted reverse cholesterol transport because it represents the first step in cellular cholesterol clearance, as only FC is effluxed from cells to HDL. Cytoplasmic lipid droplets move within the cell utilizing the cytoskeletal network, but, little is known about the influence of the cytoskeleton on lipid droplet formation. To understand this role we employed cytochalasin D (cyt.D) to promote actin depolymerization in J774 macrophages. Incubating J774 with acetylated LDL creates foam cells having a 4-fold increase in cellular cholesterol content (30-40% cholesterol present as cholesteryl ester (CE)) in cytoplasmic droplets. Lipid droplets formed in the presence of cyt.D are smaller in diameter. CE-deposition and -hydrolysis are decreased when cells are cholesterol-enriched in the presence of cyt.D or latrunculin A, another cytoskeleton disrupting agent. However, when lipid droplets formed in the presence of cyt.D are isolated and incubated with an exogenous CE hydrolase, the CE is more rapidly metabolized compared to droplets from control cells. This is apparently due to the smaller size and altered lipid composition of the droplets formed in the presence of cyt.D. Cytoskeletal proteins found on CE droplets influence droplet lipid composition and maturation in model foam cells. In J774 macrophages, cytoskeletal proteins are apparently involved in facilitating the interaction of lipid droplets and a cytosolic neutral CE hydrolase and may play a role in foam cell formation. This article is part of a Special Issue entitled Advances in High Density Lipoprotein Formation and Metabolism: A Tribute to John F. Oram (1945-2010).
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
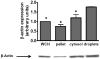


References
-
- Tang C, Oram JF. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim Biophys Acta. 2009;1791:563–572. - PubMed
-
- Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. - PubMed
-
- Oram JF, Vaughan AM. Atp-binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. - PubMed
-
- Oram JF. Hdl apolipoproteins and ABCA1: Partners in the removal of excell cellular cholesterol. Arterioscler Thromb Vasc Biol. 2003;23:720–727. - PubMed
-
- Brown MS, Ho YK, Goldstein JL. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980;255:9344–9352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

