A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome
- PMID: 22015451
- PMCID: PMC4110463
- DOI: 10.1093/annonc/mdr490
A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome
Abstract
Background: Tumor lysis syndrome (TLS) is a life-threatening disorder characterized by hyperuricemia and metabolic derangements. The efficacy of rasburicase, administered daily for 5 days, has been well established. However, the optimal duration of therapy is unknown in adults.
Patients and methods: We evaluated the efficacy of rasburicase (0.15 mg/kg) administered as single dose followed by as needed dosing (maximum five doses) versus daily dosing for 5 days in adult patients at risk for TLS.
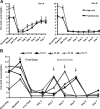
Results: Eighty of the 82 patients enrolled received rasburicase; 40 high risk [median uric acid (UA) 8.5 mg/dl; range, 1.5-19.7] and 40 potential risk (UA = 5.6 mg/dl; range, 2.4-7.4). Seventy-nine patients (99%) experienced normalization in their UA within 4 h after the first dose; 84% to an undetectable level (<0.7 mg/dl). Thirty-nine of 40 (98%) patients in the daily-dose arm and 34 of 40 (85%) patients in single-dose arm showed sustained UA response. Six high-risk patients within the single-dose arm required second dose for UA >7.5 mg/dl. Rasburicase was well tolerated; one patient with glucose-6-phosphate dehydrogenase deficiency developed methemoglobinemia and hemolysis.
Conclusions: Rasburicase is highly effective for prevention and management of hyperuricemia in adults at risk for TLS. Single-dose rasburicase was effective in most patients; only a subset of high-risk patients required a second dose.
Figures
References
-
- Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin’s lymphoma. Am J Med. 1993;94:133–139. - PubMed
-
- Hochberg J, Cairo MS. Tumor lysis syndrome: current perspective. Haematologica. 2008;93:9–13. - PubMed
-
- Mughal TI, Ejaz AA, Foringer JR, Coifffier B. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010;36:164–176. - PubMed
-
- Shimada M, Johnson RJ, May WS, Jr, et al. A novel role for uric acid in acute kidney injury associated with tumor lysis syndrome. Nephrol Dial Transplant. 2009;24:2960–2964. - PubMed
-
- Coiffer B, Altman A, Pui C-H, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767–2778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

