Tyrosine nitration of prostacyclin synthase is associated with enhanced retinal cell apoptosis in diabetes
- PMID: 22015457
- PMCID: PMC3260825
- DOI: 10.1016/j.ajpath.2011.08.041
Tyrosine nitration of prostacyclin synthase is associated with enhanced retinal cell apoptosis in diabetes
Abstract
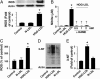
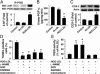
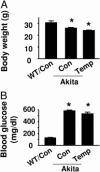
The risk of diabetic retinopathy is associated with the presence of both oxidative stress and toxic eicosanoids. Whether oxidative stress actually causes diabetic retinopathy via the generation of toxic eicosanoids, however, remains unknown. The aim of the present study was to determine whether tyrosine nitration of prostacyclin synthase (PGIS) contributes to retinal cell death in vitro and in vivo. Exposure of human retinal pericytes to heavily oxidized and glycated LDL (HOG-LDL), but not native forms of LDL (N-LDL), for 24 hours significantly increased pericyte apoptosis, accompanied by increased tyrosine nitration of PGIS and decreased PGIS activity. Inhibition of the thromboxane receptor or cyclooxygenase-2 dramatically attenuated HOG-LDL-induced apoptosis without restoring PGIS activity. Administration of superoxide dismutase (to scavenge superoxide anions) or L-N(G)-nitroarginine methyl ester (L-NAME, a nonselective nitric oxide synthase inhibitor) restored PGIS activity and attenuated pericyte apoptosis. In Akita mouse retinas, diabetes increased intraretinal levels of oxidized LDL and glycated LDL, induced PGIS nitration, enhanced apoptotic cell death, and impaired blood-retinal barrier function. Chronic administration of tempol, a superoxide scavenger, reduced intraretinal oxidized LDL and glycated LDL levels, PGIS nitration, and retina cell apoptosis, thereby preserving the integrity of blood-retinal barriers. In conclusion, oxidized LDL-mediated PGIS nitration and associated thromboxane receptor stimulation might be important in the initiation and progression of diabetic retinopathy.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Lyons T.J., Jenkins A.J., Zheng D., Lackland D.T., McGee D., Garvey W.T., Klein R.L. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45:910–918. - PubMed
-
- Klein R., Sharrett A.R., Klein B.E., Moss S.E., Folsom A.R., Wong T.Y., Brancati F.L., Hubbard L.D., Couper D. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109:1225–1234. - PubMed
-
- Wu M., Chen Y., Wilson K., Chirindel A., Ihnat M.A., Yu Y., Boulton M.E., Szweda L.I., MA J.X., Lyons T.J. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2679–2685. - PubMed
-
- Song W., Barth J.L., Lu K., Yu Y., Huang Y., Gittinger C.K., Argraves W.S., Lyons T.J. Effects of modified low-density lipoproteins on human retinal pericyte survival. Ann NY Acad Sci. 2005;1043:390–395. - PubMed
-
- Song W., Barth J.L., Yu Y., Lu K., Dashti A., Huang Y., Gittinger C.K., Argraves W.S., Lyons T.J. Effects of oxidized and glycated LDL on gene expression in human retinal capillary pericytes. Invest Ophthalmol Vis Sci. 2005;46:2974–2982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL074399/HL/NHLBI NIH HHS/United States
- R01 HL089920/HL/NHLBI NIH HHS/United States
- HL080499/HL/NHLBI NIH HHS/United States
- 3P20RR024215-03S1/RR/NCRR NIH HHS/United States
- R01 HL079584/HL/NHLBI NIH HHS/United States
- 1P20RR024215-01/RR/NCRR NIH HHS/United States
- R01 HL096032/HL/NHLBI NIH HHS/United States
- HL096032/HL/NHLBI NIH HHS/United States
- R01 HL074399/HL/NHLBI NIH HHS/United States
- HL089920/HL/NHLBI NIH HHS/United States
- HL079584/HL/NHLBI NIH HHS/United States
- HL105157/HL/NHLBI NIH HHS/United States
- R01 HL110488/HL/NHLBI NIH HHS/United States
- R01 HL105157/HL/NHLBI NIH HHS/United States
- P20 RR024215/RR/NCRR NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

