Central nervous system fibrosis is associated with fibrocyte-like infiltrates
- PMID: 22015460
- PMCID: PMC3260835
- DOI: 10.1016/j.ajpath.2011.08.036
Central nervous system fibrosis is associated with fibrocyte-like infiltrates
Abstract
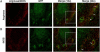
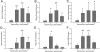
Fibrotic wall formation is essential for limiting pathogen dissemination during brain abscess development. However, little is known about the regulation of fibrotic processes in the central nervous system (CNS). Most CNS injury responses are associated with hypertrophy of resident astrocytes, a process termed reactive gliosis. Studies of fibrosis outside the CNS have identified two bone marrow-derived cell types, fibrocytes and alternatively activated M2 macrophages, as key mediators of fibrosis. The current study used bone marrow chimeras generated from green fluorescent protein transgenic mice to evaluate the appearance of these cell types and whether bone marrow-derived cells were capable of acquiring fibrotic characteristics during brain abscess development. Immunofluorescence staining revealed partial overlap between green fluorescent protein, α-smooth muscle actin, and procollagen, suggesting that a population of cells forming the brain abscess capsule originate from a bone marrow precursor. In addition, the influx of fibrocyte-like cells into brain abscesses immediately preceded the onset of fibrotic encapsulation. Fibrotic wall formation was also associated with increased numbers of alternatively activated M2 microglia and macrophages. To our knowledge, this is the first study demonstrating that bone marrow-derived infiltrates are capable of expressing fibrotic molecules during CNS inflammation.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


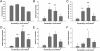
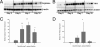

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

