Budesonide/formoterol maintenance and reliever therapy in primary care asthma management: effects on bronchial hyperresponsiveness and asthma control
- PMID: 22015542
- PMCID: PMC6548307
- DOI: 10.4104/pcrj.2011.00090
Budesonide/formoterol maintenance and reliever therapy in primary care asthma management: effects on bronchial hyperresponsiveness and asthma control
Abstract
Background: The management of asthma has changed since the introduction of budesonide/formoterol (Symbicort®) as both maintenance and reliever therapy (SMART). SMART and its effects on bronchial hyperresponsiveness (BHR) have not been studied in primary care.
Aims: To compare the effects of SMART and guideline-driven usual care (UC) on BHR and clinical asthma severity in primary care practice.
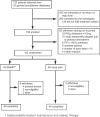
Methods: Patients with mild-to-moderate stable asthma were randomised to receive SMART treatment (n=54) (budesonide/formoterol 80/4.5 μg Turbuhaler®, two puffs once daily and extra inhalations as needed) or UC treatment (n=48) for 12 months. Diary data, Asthma Control Questionnaire scores, forced expiratory volume in 1 second (FEV1), and peak expiratory flow (PEF) measurements were collected during run-in and after 1, 3, 6, and 12 months of treatment. BHR, measured as the dose of histamine provoking a fall in FEV1 of 20% (PD20-histamine), was determined at randomisation and after 12 months.
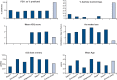
Results: One hundred and two patients with asthma participated in the study. The change in PD20-histamine during the study was not significantly different between the SMART and UC groups (p=0.26). The mean inhaled corticosteroid (ICS) dose was 326 μg beclomethasone dipropionate (BDP) equivalents/day (95% CI 254 to 399) with SMART, which was significantly lower (p<0.0001) than the mean ICS dose with UC treatment (798 μg BDP equivalents/day (95% CI 721 to 875). Morning and evening PEF values increased significantly with SMART treatment compared with UC; FEV1, symptoms and asthma control did not differ.
Conclusions: Despite a 59% lower dose of ICS, BHR and other clinical outcomes remained stable during SMART treatment while PEF values improved.
Trial registration: ClinicalTrials.gov NCT00235911.
Conflict of interest statement
RAR has received a research grant from AstraZeneca (AZ); and speaking fees, consulting fees, and reimbursement for attending conferences from AZ, GlaxoSmithKline (GSK), Boehringer, and MSD. DP has received a research grant from AZ; speaking fees, consulting fees, and reimbursement for attending conferences from AZ, GSK, MSD, Novartis, and Nycomed; funding for research from AZ, GSK, and Nycomed; travel to the ERS or ATS partially funded by AZ, GSK, Chiesi, and Nycomed; and she has been consultant to AZ, Boehringer Ingelheim, Chiesi, GSK, Nycomed, and TEVA. TvdM has received research grants from AZ, Nicomed, and MSD; speaking fees, consulting fees, and reimbursement for attending conferences from AZ and GSK; and has been consultant to AZ, MSD, Nicomed, and Novartis.
Figures


Comment in
-
Single inhaler maintenance and reliever therapy (SMART) in general practice asthma management: where are we?Prim Care Respir J. 2012 Mar;21(1):8-10. doi: 10.4104/pcrj.2012.00022. Prim Care Respir J. 2012. PMID: 22367253 Free PMC article. No abstract available.
References
-
- Global Initiative for Asthma (GINA). Global strategy for Asthma Management and Prevention (updated 2004). NIH Publication No. 02–3659. Bethesda, MD: National Institutes of Health, 2004.
-
- Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) Study. Eur Respir J 2000;16:802–7. http://dx.doi.org/10.1183/09031936.00.16580200 - PubMed
-
- Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of patients with asthma on regular maintenance therapy: the INSPIRE study. BMC Pulm Med 2006;6:13. http://dx.doi.org/10.1186/1471-2466-6-13 - PMC - PubMed
-
- Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 1997;337:1412–18. http://dx.doi.org/10.1056/NEJM199711133372001 - PubMed
-
- O'Byrne PM, Barnes PJ, Rodriguez Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164:1392–7. - PubMed
