Bax dimerizes via a symmetric BH3:groove interface during apoptosis
- PMID: 22015607
- PMCID: PMC3307980
- DOI: 10.1038/cdd.2011.138
Bax dimerizes via a symmetric BH3:groove interface during apoptosis
Abstract
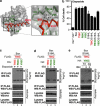
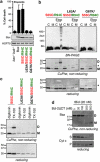
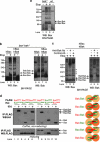
During apoptotic cell death, Bax and Bak change conformation and homo-oligomerize to permeabilize mitochondria. We recently reported that Bak homodimerizes via an interaction between the BH3 domain and hydrophobic surface groove, that this BH3:groove interaction is symmetric, and that symmetric dimers can be linked via the α6-helices to form the high order oligomers thought responsible for pore formation. We now show that Bax also dimerizes via a BH3:groove interaction after apoptotic signaling in cells and in mitochondrial fractions. BH3:groove dimers of Bax were symmetric as dimers but not higher order oligomers could be linked by cysteine residues placed in both the BH3 and groove. The BH3:groove interaction was evident in the majority of mitochondrial Bax after apoptotic signaling, and correlated strongly with cytochrome c release, supporting its central role in Bax function. A second interface between the Bax α6-helices was implicated by cysteine linkage studies, and could link dimers to higher order oligomers. We also found that a population of Bax:Bak heterodimers generated during apoptosis formed via a BH3:groove interaction, further demonstrating that Bax and Bak oligomerize via similar mechanisms. These findings highlight the importance of BH3:groove interactions in apoptosis regulation by the Bcl-2 protein family.
Figures



References
-
- Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. - PubMed
-
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. - PubMed
-
- Hsu Y-T, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

