Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques
- PMID: 22015609
- PMCID: PMC3307982
- DOI: 10.1038/cdd.2011.140
Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques
Abstract
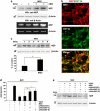
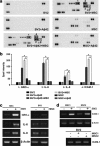
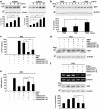
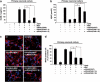
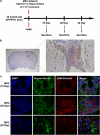
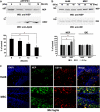
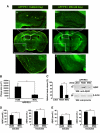
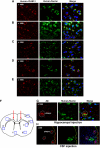
Presently, co-culture of human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) with BV2 microglia under amyloid-β42 (Aβ42) exposure induced a reduction of Aβ42 in the medium as well as an overexpression of the Aβ-degrading enzyme neprilysin (NEP) in microglia. Cytokine array examinations of co-cultured media revealed elevated release of soluble intracellular adhesion molecule-1 (sICAM-1) from hUCB-MSCs. Administration of human recombinant ICAM-1 in BV2 cells and wild-type mice brains induced NEP expression in time- and dose-dependent manners. In co-culturing with BV2 cells under Aβ42 exposure, knockdown of ICAM-1 expression on hUCB-MSCs by small interfering RNA (siRNA) abolished the induction of NEP in BV2 cells as well as reduction of added Aβ42 in the co-cultured media. By contrast, siRNA-mediated inhibition of the sICAM-1 receptor, lymphocyte function-associated antigen-1 (LFA-1), on BV2 cells reduced NEP expression by ICAM-1 exposure. When hUCB-MSCs were transplanted into the hippocampus of a 10-month-old transgenic mouse model of Alzheimer's disease for 10, 20, or 40 days, NEP expression was increased in the mice brains. Moreover, Aβ42 plaques in the hippocampus and other regions were decreased by active migration of hUCB-MSCs toward Aβ deposits. These data suggest that hUCB-MSC-derived sICAM-1 decreases Aβ plaques by inducing NEP expression in microglia through the sICAM-1/LFA-1 signaling pathway.
Figures
References
-
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. - PubMed
-
- Miller G. Pharmacology. The puzzling rise and fall of a dark-horse Alzheimer's drug. Science. 2010;327:1309. - PubMed
-
- Secco M, Zucconi E, Vieira NM, Fogaca LL, Cerqueira A, Carvalho MD, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146–150. - PubMed
-
- Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251:116–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

