Isolated in vitro brainstem-spinal cord preparations remain important tools in respiratory neurobiology
- PMID: 22015642
- PMCID: PMC3242829
- DOI: 10.1016/j.resp.2011.10.002
Isolated in vitro brainstem-spinal cord preparations remain important tools in respiratory neurobiology
Abstract
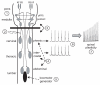
Isolated in vitro brainstem-spinal cord preparations are used extensively in respiratory neurobiology because the respiratory network in the pons and medulla is intact, monosynaptic descending inputs to spinal motoneurons can be activated, brainstem and spinal cord tissue can be bathed with different solutions, and the responses of cervical, thoracic, and lumbar spinal motoneurons to experimental perturbations can be compared. The caveats and limitations of in vitro brainstem-spinal cord preparations are well-documented. However, isolated brainstem-spinal cords are still valuable experimental preparations that can be used to study neuronal connectivity within the brainstem, development of motor networks with lethal genetic mutations, deleterious effects of pathological drugs and conditions, respiratory spinal motor plasticity, and interactions with other motor behaviors. Our goal is to show how isolated brainstem-spinal cord preparations still have a lot to offer scientifically and experimentally to address questions within and outside the field of respiratory neurobiology.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

References
-
- Abadie V, Champagnat J, Fortin G. Branchiomotor activities in mouse embryo. Neuroreport. 2000;11:141–145. - PubMed
-
- Barnes BJ, Tuong CM, Mellen NM. Functional imaging reveals respiratory network activity during hypoxic and opioid challenge in the neonate rat tilted sagittal slab preparation. J Neurophysiol. 2007;97:2283–2292. - PubMed
-
- Berner J, Shvarev Y, Lagercrantz H, Bilkei-Gorzo A, Hökfelt T, Wickström R. Altered respiratory pattern and hypoxic response in transgenic newborn mice lacking the tachykinin-1 gene. J Appl Physiol. 2007;103:552–559. - PubMed
-
- Borday C, Abadie V, Chatonnet F, Thoby-Brisson M, Champagnat J, Fortin G. Developmental molecular switches regulating breathing patterns in CNS. Respir Physiol Neurobiol. 2003;135:121–132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

