Lysophosphatidic acid signaling protects pulmonary vasculature from hypoxia-induced remodeling
- PMID: 22015657
- PMCID: PMC3241874
- DOI: 10.1161/ATVBAHA.111.234708
Lysophosphatidic acid signaling protects pulmonary vasculature from hypoxia-induced remodeling
Abstract
Objective: Lysophosphatidic acid (LPA) is a bioactive lipid molecule produced by the plasma lysophospholipase D enzyme autotaxin that is present at ≥100 nmol/L in plasma. Local administration of LPA promotes systemic arterial remodeling in rodents. To determine whether LPA contributes to remodeling of the pulmonary vasculature, we examined responses in mice with alterations in LPA signaling and metabolism.
Methods and results: Enpp2(+/-) mice, which are heterozygous for the autotaxin-encoding gene and which have reduced expression of autotaxin/lysophospholipase D and approximately half normal plasma LPA, were hyperresponsive to hypoxia-induced vasoconstriction and remodeling, as evidenced by the development of higher right ventricular (RV) systolic pressure, greater decline in peak flow velocity across the pulmonary valve, and a higher percentage of muscularized arterioles. Mice lacking LPA(1) and LPA(2), 2 LPA receptors abundantly expressed in the vasculature, also had enhanced hypoxia-induced pulmonary remodeling. With age, Lpar1(-/-)2(-/-) mice spontaneously developed elevated RV systolic pressure and RV hypertrophy that was not observed in Lpar1(-/-) mice or Lpar2(-/-) mice. Expression of endothelin-1, a potent vasoconstrictor, was elevated in lungs of Lpar1(-/-)2(-/-) mice, and expression of endothelin(B) receptor, which promotes vasodilation and clears endothelin, was reduced in Enpp2(+/-) and Lpar1(-/-)2(-/-) mice.
Conclusions: Our findings indicate that LPA may negatively regulate pulmonary vascular pressure through LPA(1) and LPA(2) receptors and that in the absence of LPA signaling, upregulation in the endothelin system favors remodeling.
Conflict of interest statement
The authors have no first-tier potential conflicts of interest with the submitted work to report. SSS has received investigator-initiated research/grant support from AstraZeneca, Boehringer Ingelheim, and The Medicines Company in excess of $50,000 for unrelated work, and her laboratory serves as a core laboratory for pharmacodynamic analysis overseen by CirQuest Laboratories that is part of a preplanned substudy of the TRACER trial.
Figures
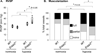

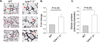


References
-
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004 Sep 30;351(14):1425–1436. - PubMed
-
- McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006 Sep 26;114(13):1417–1431. - PubMed
-
- De Marco T. Pulmonary arterial hypertension and women. Cardiol Rev. 2006 Nov–Dec;14(6):312–318. - PubMed
-
- Gaine S. Pulmonary hypertension. Jama. 2000 Dec 27;284(24):3160–3168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

