Role of telomeres and telomerase in cancer
- PMID: 22015685
- PMCID: PMC3370415
- DOI: 10.1016/j.semcancer.2011.10.001
Role of telomeres and telomerase in cancer
Abstract
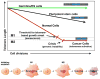
There is mounting evidence for the existence of an important relationship between telomeres and telomerase and cellular aging and cancer. Normal human cells progressively lose telomeres with each cell division until a few short telomeres become uncapped leading to a growth arrest known as replicative aging. In the absence of genomic alterations these cells do not die but remain quiescent producing a different constellation of proteins compared to young quiescent cells. Upon specific genetic and epigenetic alterations, normal human cells bypass replicative senescence and continue to proliferate until many telomere ends become uncapped leading to a phenomenon known as crisis. In crisis cells have critically shortened telomeres but continue to attempt to divide leading to significant cell death (apoptosis) and progressive genomic instability. Rarely, a human cell escapes crisis and these cells almost universally express the ribonucleoprotein, telomerase, and maintain stable but short telomeres. The activation of telomerase may be thought of as a mechanism to slow down the rate genomic instability due to dysfunctional telomeres. While telomerase does not drive the oncogenic process, it is permissive and required for the sustain growth of most advanced cancers. Since telomerase is not expressed in most normal human cells, this has led to the development of targeted telomerase cancer therapeutic approaches that are presently in advanced clinical trials.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–662. - PubMed
-
- Hayflick L. How and why we age. Exp Gerontol. 1998;33:639–65. - PubMed
-
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36. - PubMed
-
- Hayflick L. The coming of age of WI-38. Adv Cell Cult. 1984;3:303–16.
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

