Histone chaperones link histone nuclear import and chromatin assembly
- PMID: 22015777
- PMCID: PMC3272145
- DOI: 10.1016/j.bbagrm.2011.09.007
Histone chaperones link histone nuclear import and chromatin assembly
Abstract
Histone chaperones are proteins that shield histones from nonspecific interactions until they are assembled into chromatin. After their synthesis in the cytoplasm, histones are bound by different histone chaperones, subjected to a series of posttranslational modifications and imported into the nucleus. These evolutionarily conserved modifications, including acetylation and methylation, can occur in the cytoplasm, but their role in regulating import is not well understood. As part of histone import complexes, histone chaperones may serve to protect the histones during transport, or they may be using histones to promote their own nuclear localization. In addition, there is evidence that histone chaperones can play an active role in the import of histones. Histone chaperones have also been shown to regulate the localization of important chromatin modifying enzymes. This review is focused on the role histone chaperones play in the early biogenesis of histones, the distinct cytoplasmic subcomplexes in which histone chaperones have been found in both yeast and mammalian cells and the importins/karyopherins and nuclear localization signals that mediate the nuclear import of histones. We also address the role that histone chaperone localization plays in human disease. This article is part of a Special Issue entitled: Histone chaperones and chromatin assembly.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
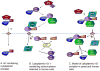
Similar articles
-
Histone chaperones link histone nuclear import and chromatin assembly.Biochim Biophys Acta. 2013 Mar-Apr;1819(3-4):277-89. Biochim Biophys Acta. 2013. PMID: 24459730 Review.
-
Nuclear import of histones.Biochem Soc Trans. 2020 Dec 18;48(6):2753-2767. doi: 10.1042/BST20200572. Biochem Soc Trans. 2020. PMID: 33300986 Free PMC article. Review.
-
Nap1 and Chz1 have separate Htz1 nuclear import and assembly functions.Traffic. 2010 Feb;11(2):185-97. doi: 10.1111/j.1600-0854.2009.01010.x. Epub 2009 Nov 19. Traffic. 2010. PMID: 19929865 Free PMC article.
-
Yeast chromatin assembly complex 1 protein excludes nonacetylatable forms of histone H4 from chromatin and the nucleus.Mol Cell Biol. 2004 Dec;24(23):10180-92. doi: 10.1128/MCB.24.23.10180-10192.2004. Mol Cell Biol. 2004. PMID: 15542829 Free PMC article.
-
Nuclear import of histone H2A and H2B is mediated by a network of karyopherins.J Cell Biol. 2001 Apr 16;153(2):251-62. doi: 10.1083/jcb.153.2.251. J Cell Biol. 2001. PMID: 11309407 Free PMC article.
Cited by
-
Mechanistic insights into histone deposition and nucleosome assembly by the chromatin assembly factor-1.Nucleic Acids Res. 2018 Nov 2;46(19):9907-9917. doi: 10.1093/nar/gky823. Nucleic Acids Res. 2018. PMID: 30239791 Free PMC article. Review.
-
Chaperoning the chaperones: Proteomic analysis of the SMN complex reveals conserved and etiologic connections to the proteostasis network.bioRxiv [Preprint]. 2024 May 16:2024.05.15.594402. doi: 10.1101/2024.05.15.594402. bioRxiv. 2024. Update in: Front RNA Res. 2024;2:1448194. doi: 10.3389/frnar.2024.1448194. PMID: 38903116 Free PMC article. Updated. Preprint.
-
Motifs in the amino-terminus of CENP-A are required for its accumulation within the nucleus and at the centromere.Oncotarget. 2017 Jun 20;8(25):40654-40667. doi: 10.18632/oncotarget.17204. Oncotarget. 2017. PMID: 28489565 Free PMC article.
-
HAT1 Coordinates Histone Production and Acetylation via H4 Promoter Binding.Mol Cell. 2019 Aug 22;75(4):711-724.e5. doi: 10.1016/j.molcel.2019.05.034. Epub 2019 Jul 2. Mol Cell. 2019. PMID: 31278053 Free PMC article.
-
Characterization of two different Asf1 histone chaperones with distinct cellular localizations and functions in Trypanosoma brucei.Nucleic Acids Res. 2014 Mar;42(5):2906-18. doi: 10.1093/nar/gkt1267. Epub 2013 Dec 9. Nucleic Acids Res. 2014. PMID: 24322299 Free PMC article.
References
-
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. - PubMed
-
- Nakagawa T, Bulger M, Muramatsu M, Ito T. Multistep chromatin assembly on supercoiled plasmid DNA by nucleosome assembly protein-1 and ATP-utilizing chromatin assembly and remodeling factor. J Biol Chem. 2001;276:27384–27391. - PubMed
-
- Annunziato AT. Assembling chromatin: The long and winding road. Biochim Biophys Acta. 2011 This issue. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

