Aspirin and heparin effect on basal and antiphospholipid antibody modulation of trophoblast function
- PMID: 22015869
- PMCID: PMC3199133
- DOI: 10.1097/AOG.0b013e31823234ad
Aspirin and heparin effect on basal and antiphospholipid antibody modulation of trophoblast function
Abstract
Objective: Low molecular weight (LMW) heparin, with or without aspirin (acetylsalicylic acid [ASA]), is used to prevent complications in antiphospholipid syndrome in pregnancy. Our objective was to elucidate the actions of low-dose LMW heparin and ASA on basal and antiphospholipid antibody-induced modulation of trophoblast function.
Methods: The human first-trimester trophoblast cell line (HTR-8) was treated with or without antiphospholipid antibody in the presence of no medication, low-dose LMW heparin, low-dose ASA, or combination therapy. Interleukin (IL)-6, IL-8, IL-1β, growth-regulated oncogene-α, vascular endothelial growth factor (VEGF), placental growth factor, soluble FMS-like tyrosine kinase-1, and soluble endoglin were measured in the supernatant. Cell migration was performed using a two-chamber assay.
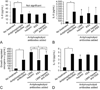
Results: Low molecular weight heparin improved basal trophoblast migration and induced potent increases in growth-regulated oncogene-α and soluble FMS-like tyrosine kinase-1. Aspirin did not affect basal function. Combined therapy promoted migration but did not reverse the LMW heparin-induced soluble FMS-like tyrosine kinase-1 effect. Antiphospholipid antibody increased IL-8, IL-1β, growth-regulated oncogene-alpha, VEGF, placental growth factor, and soluble endoglin secretion, while decreasing cell migration and IL-6 and soluble FMS-like tyrosine kinase-1 secretion. The antiphospholipid antibody-induced cytokine changes were best reversed with LMW heparin, with partial reversal of IL-8 and IL-1β upregulation. The antiphospholipid antibody-induced angiogenic changes were worsened by LMW heparin, with increased soluble FMS-like tyrosine kinase-1 secretion. The therapies did not reverse antiphospholipid antibody-induced decrease in migration.
Conclusion: In the absence of antiphospholipid antibodies, LMW heparin induces potentially detrimental proinflammatory and antiangiogenic profile in the trophoblast. In the presence of antiphospholipid antibodies, single-agent LMW heparin may be the optimal therapy to counter trophoblast inflammation, but also induces an antiangiogenic response. These findings may explain the inability of current therapies to consistently prevent adverse outcomes.
Figures





References
-
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. - PubMed
-
- Denney JM, Porter TF, Branch DW. Autoimmune Diseases. In: David James, Philip J. Steer, Carl P. Weiner, Bernard Gonik., editors. High Risk Pregnancy: Management Options. New York, NY: Saunders; 2011. p. 763.
-
- Backos M, Rai R, Baxter N, Chilcott IT, Cohen H, Regan L. Pregnancy complications in women with recurrent miscarriage associated with antiphospholipid antibodies treated with low-dose aspirin and heparin. Br J Obstet Gynaecol. 1999;106:102–107. - PubMed
-
- Branch DW, Khamashta MA. Antiphospholipid syndrome: Obstetric diagnosis, management, and controversies. Obstet Gynecol. 2003;101:1333–1344. - PubMed
-
- Stephenson MD, Ballem PJ, Tsang P, Purkiss S, Ensworth S, Houlihan E, et al. Treatment of antiphospholipid antibody syndrome (APS) in pregnancy: A randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. J Obstet Gynaecol can. 2004;26:729–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

