A hypothesis for basal ganglia-dependent reinforcement learning in the songbird
- PMID: 22015923
- PMCID: PMC3221789
- DOI: 10.1016/j.neuroscience.2011.09.069
A hypothesis for basal ganglia-dependent reinforcement learning in the songbird
Erratum in
- Neuroscience. 2013 Dec 26;255:301
Abstract
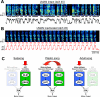
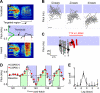
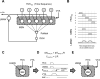
Most of our motor skills are not innately programmed, but are learned by a combination of motor exploration and performance evaluation, suggesting that they proceed through a reinforcement learning (RL) mechanism. Songbirds have emerged as a model system to study how a complex behavioral sequence can be learned through an RL-like strategy. Interestingly, like motor sequence learning in mammals, song learning in birds requires a basal ganglia (BG)-thalamocortical loop, suggesting common neural mechanisms. Here, we outline a specific working hypothesis for how BG-forebrain circuits could utilize an internally computed reinforcement signal to direct song learning. Our model includes a number of general concepts borrowed from the mammalian BG literature, including a dopaminergic reward prediction error and dopamine-mediated plasticity at corticostriatal synapses. We also invoke a number of conceptual advances arising from recent observations in the songbird. Specifically, there is evidence for a specialized cortical circuit that adds trial-to-trial variability to stereotyped cortical motor programs, and a role for the BG in "biasing" this variability to improve behavioral performance. This BG-dependent "premotor bias" may in turn guide plasticity in downstream cortical synapses to consolidate recently learned song changes. Given the similarity between mammalian and songbird BG-thalamocortical circuits, our model for the role of the BG in this process may have broader relevance to mammalian BG function.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. I. Physiological properties of striatal microexcitable zones. J Neurophysiol. 1985;53:1401–1416. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

