Administration of thimerosal to infant rats increases overflow of glutamate and aspartate in the prefrontal cortex: protective role of dehydroepiandrosterone sulfate
- PMID: 22015977
- PMCID: PMC3264864
- DOI: 10.1007/s11064-011-0630-z
Administration of thimerosal to infant rats increases overflow of glutamate and aspartate in the prefrontal cortex: protective role of dehydroepiandrosterone sulfate
Abstract
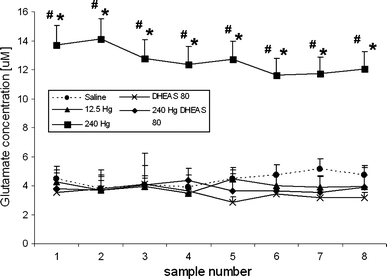
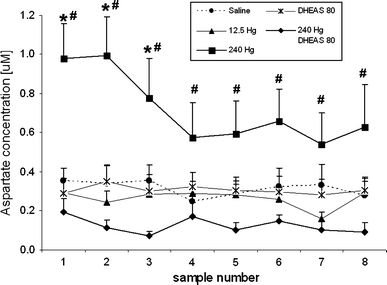
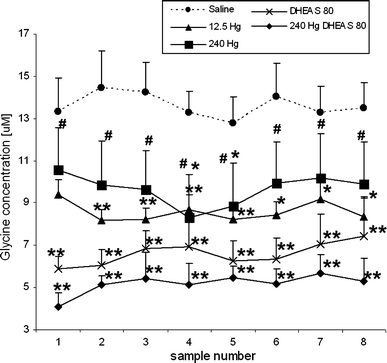
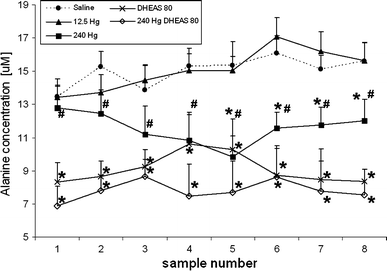
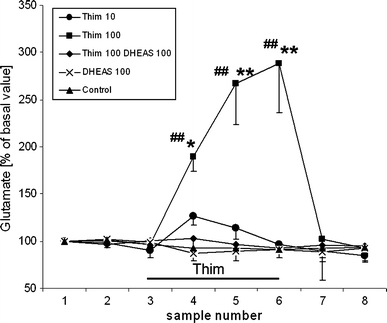
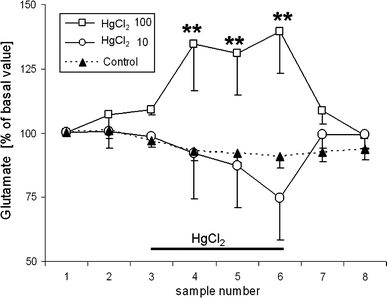
Thimerosal, a mercury-containing vaccine preservative, is a suspected factor in the etiology of neurodevelopmental disorders. We previously showed that its administration to infant rats causes behavioral, neurochemical and neuropathological abnormalities similar to those present in autism. Here we examined, using microdialysis, the effect of thimerosal on extracellular levels of neuroactive amino acids in the rat prefrontal cortex (PFC). Thimerosal administration (4 injections, i.m., 240 μg Hg/kg on postnatal days 7, 9, 11, 15) induced lasting changes in amino acid overflow: an increase of glutamate and aspartate accompanied by a decrease of glycine and alanine; measured 10-14 weeks after the injections. Four injections of thimerosal at a dose of 12.5 μg Hg/kg did not alter glutamate and aspartate concentrations at microdialysis time (but based on thimerosal pharmacokinetics, could have been effective soon after its injection). Application of thimerosal to the PFC in perfusion fluid evoked a rapid increase of glutamate overflow. Coadministration of the neurosteroid, dehydroepiandrosterone sulfate (DHEAS; 80 mg/kg; i.p.) prevented the thimerosal effect on glutamate and aspartate; the steroid alone had no influence on these amino acids. Coapplication of DHEAS with thimerosal in perfusion fluid also blocked the acute action of thimerosal on glutamate. In contrast, DHEAS alone reduced overflow of glycine and alanine, somewhat potentiating the thimerosal effect on these amino acids. Since excessive accumulation of extracellular glutamate is linked with excitotoxicity, our data imply that neonatal exposure to thimerosal-containing vaccines might induce excitotoxic brain injuries, leading to neurodevelopmental disorders. DHEAS may partially protect against mercurials-induced neurotoxicity.
Figures
References
-
- Qvarnström J, Lambertsson L, Havarinasab S, Hultman P, Frech W. Determination of methylmercury, ethylmercury, and inorganic mercury in mouse tissues, following administration of thimerosal, by species-specific isotope dilution GC-inductively coupled plasma-MS. Anal Chem. 2003;75:4120–4124. doi: 10.1021/ac0342370. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

