Synthetic Escherichia coli consortia engineered for syntrophy demonstrate enhanced biomass productivity
- PMID: 22015987
- PMCID: PMC3549274
- DOI: 10.1016/j.jbiotec.2011.10.001
Synthetic Escherichia coli consortia engineered for syntrophy demonstrate enhanced biomass productivity
Abstract
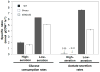
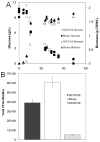
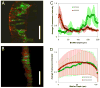
Synthetic Escherichia coli consortia engineered for syntrophy demonstrated enhanced biomass productivity relative to monocultures. Binary consortia were designed to mimic a ubiquitous, naturally occurring ecological template of primary productivity supported by secondary consumption. The synthetic consortia replicated this evolution-proven strategy by combining a glucose positive E. coli strain, which served as the system's primary producer, with a glucose negative E. coli strain which consumed metabolic byproducts from the primary producer. The engineered consortia utilized strategic division of labor to simultaneously optimize multiple tasks enhancing overall culture performance. Consortial interactions resulted in the emergent property of enhanced system biomass productivity which was demonstrated with three distinct culturing systems: batch, chemostat and biofilm growth. Glucose-based biomass productivity increased by ∼15, 20 and 50% compared to appropriate monoculture controls for these three culturing systems, respectively. Interestingly, the consortial interactions also produced biofilms with predictable, self-assembling, laminated microstructures. This study establishes a metabolic engineering paradigm which can be easily adapted to existing E. coli based bioprocesses to improve productivity based on a robust ecological theme.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures





References
-
- Aristidou AA, et al. Modification of central metabolic pathway in escherichia coli to reduce acetate accumulation by heterologous expression of the bacillus subtilis acetolactate synthase gene. Biotechnol Bioeng. 1994;44:944–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

