T cell and B Cell immunity can be reconstituted with mismatched hematopoietic stem cell transplantation without alkylator therapy in artemis-deficient mice using anti-natural killer cell antibody and photochemically treated sensitized donor T cells
- PMID: 22015994
- PMCID: PMC3258350
- DOI: 10.1016/j.bbmt.2011.10.017
T cell and B Cell immunity can be reconstituted with mismatched hematopoietic stem cell transplantation without alkylator therapy in artemis-deficient mice using anti-natural killer cell antibody and photochemically treated sensitized donor T cells
Abstract
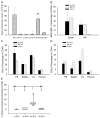
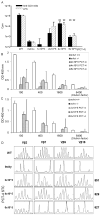
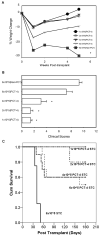
Children with Artemis-deficient T(-)B(-)NK(+) severe combined immunodeficiency are at high risk for graft rejection from natural killer (NK) cells and toxicity from increased sensitivity to the alkylating agents used in mismatched hematopoietic stem cell transplantation (HSCT). We evaluated the use of a nonalkylating agent regimen before HSCT in Artemis-deficient (mArt(-/-)) C57Bl/6 (B6) mice to open marrow niches and achieve long-term multilineage engraftment with full T cell and B cell immune reconstitution. We found that partial depletion of both recipient NK cells using anti-NK1.1 monoclonal antibody and donor T cells sensitized to recipient splenocytes was necessary. BALB/c-sensitized T cells (STCs) were photochemically treated (PCT) with psoralen and UVA light to inhibit proliferation, reduce the risk of graft-versus-host disease (GVHD), and target host hematopoietic stem cells (HSCs). A dose of 4 × 10(5) PCT STCs coinjected with 1 × 10(5) lineage-depleted c-kit(+) BALB/c HSCs resulted in 43.9% ± 3.3% CD4(+) and 10.9% ± 1.2% CD8(+) donor T cells in blood, 29% ± 7.8% and 21.7% ± 4.0 donor B220(+) IgM(+) in spleen and bone marrow, and 15.0% ± 3.6% donor Gran-1(+) cells in bone marrow at 6 months post-HSCT versus 0.02% ± 0.01%, 0.13% ± 0.10%, 0.53% ± 0.16%, 0.49% ± 0.09%, and 0.20% ± 0.06%, respectively, in controls who did not receive PCT STCs. We found that STCs target host HSCs and that PCT STCs are detectable only up to 24 hours after infusion, in contrast to non-photochemically treated STCs, which proliferate resulting in fatal GVHD. Increased mortality in the groups receiving 4-6 × 10(5) PCT STCs was associated with evidence of GVHD, particularly in the recipients of 6 × 10(5) cells. These results demonstrate that blocking NK cell-mediated resistance and making niches in bone marrow are both essential to achieving multilineage engraftment of mismatched donor cells and T cell and B cell reconstitution, even though GVHD is not completely eliminated.
Copyright © 2012 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. - PubMed
-
- O'Marcaigh AS, DeSantes K, Hu D, Pabst H, Horn B, et al. Bone marrow transplantation for T-B- severe combined immunodeficiency disease in Athabascan-speaking native Americans. Bone Marrow Transplant. 2001;27:703–709. - PubMed
-
- Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

