Time-dependent processes in stem cell-based tissue engineering of articular cartilage
- PMID: 22016073
- PMCID: PMC3412929
- DOI: 10.1007/s12015-011-9328-5
Time-dependent processes in stem cell-based tissue engineering of articular cartilage
Abstract
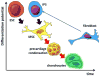
Articular cartilage (AC), situated in diarthrodial joints at the end of the long bones, is composed of a single cell type (chondrocytes) embedded in dense extracellular matrix comprised of collagens and proteoglycans. AC is avascular and alymphatic and is not innervated. At first glance, such a seemingly simple tissue appears to be an easy target for the rapidly developing field of tissue engineering. However, cartilage engineering has proven to be very challenging. We focus on time-dependent processes associated with the development of native cartilage starting from stem cells, and the modalities for utilizing these processes for tissue engineering of articular cartilage.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures



Similar articles
-
Mechanisms of synovial joint and articular cartilage development.Cell Mol Life Sci. 2019 Oct;76(20):3939-3952. doi: 10.1007/s00018-019-03191-5. Epub 2019 Jun 14. Cell Mol Life Sci. 2019. PMID: 31201464 Free PMC article. Review.
-
Stem cells for tissue engineering of articular cartilage.Proc Inst Mech Eng H. 2007 Jul;221(5):441-50. doi: 10.1243/09544119JEIM257. Proc Inst Mech Eng H. 2007. PMID: 17822146 Review.
-
Genesis and morphogenesis of limb synovial joints and articular cartilage.Matrix Biol. 2014 Oct;39:5-10. doi: 10.1016/j.matbio.2014.08.006. Epub 2014 Aug 27. Matrix Biol. 2014. PMID: 25172830 Free PMC article. Review.
-
Mechanobioreactors for Cartilage Tissue Engineering.Methods Mol Biol. 2015;1340:203-19. doi: 10.1007/978-1-4939-2938-2_15. Methods Mol Biol. 2015. PMID: 26445841
-
Functional tissue-engineered microtissue derived from cartilage extracellular matrix for articular cartilage regeneration.Acta Biomater. 2018 Sep 1;77:127-141. doi: 10.1016/j.actbio.2018.07.031. Epub 2018 Jul 18. Acta Biomater. 2018. PMID: 30030172
Cited by
-
Micro- and nanotechnology in biomedical engineering for cartilage tissue regeneration in osteoarthritis.Beilstein J Nanotechnol. 2022 Apr 11;13:363-389. doi: 10.3762/bjnano.13.31. eCollection 2022. Beilstein J Nanotechnol. 2022. PMID: 35529803 Free PMC article. Review.
-
Dynamic mechanical loading facilitated chondrogenic differentiation of rabbit BMSCs in collagen scaffolds.Regen Biomater. 2019 Mar;6(2):99-106. doi: 10.1093/rb/rbz005. Epub 2019 Feb 4. Regen Biomater. 2019. PMID: 30967964 Free PMC article.
-
A developmentally inspired combined mechanical and biochemical signaling approach on zonal lineage commitment of mesenchymal stem cells in articular cartilage regeneration.Integr Biol (Camb). 2015 Jan;7(1):112-27. doi: 10.1039/c4ib00197d. Integr Biol (Camb). 2015. PMID: 25387395 Free PMC article.
-
Challenges in engineering osteochondral tissue grafts with hierarchical structures.Expert Opin Biol Ther. 2015;15(11):1583-99. doi: 10.1517/14712598.2015.1070825. Epub 2015 Jul 20. Expert Opin Biol Ther. 2015. PMID: 26195329 Free PMC article. Review.
-
Effect of Conditioned Medium from IGF1-Induced Human Wharton's Jelly Mesenchymal Stem Cells (IGF1-hWJMSCs-CM) on Osteoarthritis.Avicenna J Med Biotechnol. 2020 Jul-Sep;12(3):172-178. Avicenna J Med Biotechnol. 2020. PMID: 32695280 Free PMC article.
References
-
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. The New England Journal of Medicine. 1994;331:889–95. - PubMed
-
- Malicev E, Barlic A, Kregar-Velikonja N, Stražar K, Drobnic M. Cartilage from the edge of a debrided articular defect is inferior to that from a standard donor site when used for autologous chondrocyte cultivation. The Journal of Bone and Joint Surgery British Volume. 2011;93:421–6. - PubMed
-
- Goessler UR, Bugert P, Bieback K, Baisch A, Sadick H, Verse T, et al. Expression of collagen and fiber-associated proteins in human septal cartilage during in vitro dedifferentiation. International Journal of Molecular Medicine. 2004;14:1015–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

