Pharmacokinetics of moxifloxacin in an infant with Mycoplasma hominis meningitis
- PMID: 22016080
- PMCID: PMC3358780
- DOI: 10.1097/INF.0b013e31823980c3
Pharmacokinetics of moxifloxacin in an infant with Mycoplasma hominis meningitis
Abstract
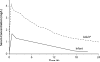
Treatment of Mycoplasma hominis meningitis in infants is limited by a lack of consensus regarding therapy and limited pharmacokinetic data for agents to which M. hominis is susceptible. We report the successful treatment of a premature infant suffering from M. hominis meningitis with doxycycline and moxifloxacin and provide a pharmacokinetic profile of moxifloxacin.
Figures
Similar articles
-
[Moxifloxacin treatment for Mycoplasma hominis meningitis in an extremely preterm infant].Zhongguo Dang Dai Er Ke Za Zhi. 2024 Apr 15;26(4):432-436. doi: 10.7499/j.issn.1008-8830.2312016. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 38660910 Free PMC article. Chinese.
-
The Pharmacokinetics of Moxifloxacin in Cerebrospinal Fluid Following Intravenous Administration: A Report of Successfully Treated Infant with Mycoplasma hominis Meningitis.Pediatr Infect Dis J. 2020 Aug;39(8):e183-e184. doi: 10.1097/INF.0000000000002655. Pediatr Infect Dis J. 2020. PMID: 32195773
-
[Mycoplasma hominis meningitis in a premature baby with hydrocephalus].Ugeskr Laeger. 2005 May 30;167(22):2416-7. Ugeskr Laeger. 2005. PMID: 15987036 Danish. No abstract available.
-
Mycoplasma hominis meningitis in a neonate: case report and review.J Infect. 2008 Oct;57(4):338-43. doi: 10.1016/j.jinf.2008.08.002. Epub 2008 Sep 14. J Infect. 2008. PMID: 18790539 Review.
-
Diagnosis and antimicrobial therapy of Mycoplasma hominis meningitis in adults.Int J Med Microbiol. 2012 Dec;302(7-8):289-92. doi: 10.1016/j.ijmm.2012.09.003. Epub 2012 Oct 22. Int J Med Microbiol. 2012. PMID: 23085510 Review.
Cited by
-
Therapeutic Drug Monitoring of Moxifloxacin to Guide Treatment of Mycoplasma hominis Meningitis in an Extremely Preterm Infant.J Pediatr Pharmacol Ther. 2021;26(8):857-862. doi: 10.5863/1551-6776-26.8.857. Epub 2021 Nov 10. J Pediatr Pharmacol Ther. 2021. PMID: 34790077 Free PMC article.
-
Mycoplasma hominis Meningitis in a Full-Term Neonate: Rapid Recovery without Specific Treatment.Indian J Pediatr. 2016 Sep;83(9):1030-1. doi: 10.1007/s12098-016-2116-0. Epub 2016 Apr 25. Indian J Pediatr. 2016. PMID: 27113078 No abstract available.
-
Pediatric anthrax clinical management.Pediatrics. 2014 May;133(5):e1411-36. doi: 10.1542/peds.2014-0563. Pediatrics. 2014. PMID: 24777226 Free PMC article.
-
Evaluation of evidence for pharmacokinetics-pharmacodynamics-based dose optimization of antimicrobials for treating Gram-negative infections in neonates.Indian J Med Res. 2017 Mar;145(3):299-316. doi: 10.4103/ijmr.IJMR_723_15. Indian J Med Res. 2017. PMID: 28749392 Free PMC article.
-
CDC Guidelines for the Prevention and Treatment of Anthrax, 2023.MMWR Recomm Rep. 2023 Nov 17;72(6):1-47. doi: 10.15585/mmwr.rr7206a1. MMWR Recomm Rep. 2023. PMID: 37963097 Free PMC article.
References
-
- Hata A, Honda Y, Asada K, et al. Mycoplasma hominis meningitis in a neonate: case report and review. J Infect. 2008;57(4):338–43. - PubMed
-
- Krausse R, Schubert S. In-vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma ssp. isolated in Germany over 20 years. Clin Microbiol Infect. 2010;16(11):1649–55. - PubMed
-
- Machida M, Kusajima H, Aijima H, et al. Toxicokinetic study of norfloxacin-induced arthropathy in juvenile animals. Toxicol Appl Pharmacol. 1990;105(3):403–12. - PubMed
-
- Wolthers KC, Kornelisse RF, Platenkamp GJ, et al. A case of Mycoplasma hominis meningoencephalitis in a full-term infant: rapid recovery after start of treatment with ciprofloxacin. Eur J Pediatr. 2003;162(7-8):514–6. - PubMed
-
- Riegelman S, Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980;8(5):509–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- T32 HD043029/HD/NICHD NIH HHS/United States
- 1K23HL092225-01/HL/NHLBI NIH HHS/United States
- K23 HD068497/HD/NICHD NIH HHS/United States
- R01 HD057956/HD/NICHD NIH HHS/United States
- 1R01HD057956-02/HD/NICHD NIH HHS/United States
- 5T32HD043029-09/HD/NICHD NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- 1R18AE000028-01/AE/ASPE HHS/United States
- 1K24HD058735-01/HD/NICHD NIH HHS/United States
- R01 FD003519/FD/FDA HHS/United States
- 1R01FD003519-01/FD/FDA HHS/United States
- K23 HD060040/HD/NICHD NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- HHSN275201000002C/HD/NICHD NIH HHS/United States
- 1K23HD064814-01/HD/NICHD NIH HHS/United States
- K23 HD064814/HD/NICHD NIH HHS/United States
- HHSN275201000002I/PHS HHS/United States
- HHSN267200700051C/DK/NIDDK NIH HHS/United States
- U10 HD045962/HD/NICHD NIH HHS/United States
- 1K23HD060040-01/HD/NICHD NIH HHS/United States
- 1U10-HD45962-06/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical