Vagal neural crest cell migratory behavior: a transition between the cranial and trunk crest
- PMID: 22016183
- PMCID: PMC4070611
- DOI: 10.1002/dvdy.22715
Vagal neural crest cell migratory behavior: a transition between the cranial and trunk crest
Abstract
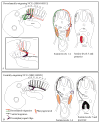
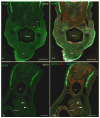
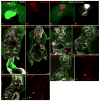
Migration and differentiation of cranial neural crest cells are largely controlled by environmental cues, whereas pathfinding at the trunk level is dictated by cell-autonomous molecular changes owing to early specification of the premigratory crest. Here, we investigated the migration and patterning of vagal neural crest cells. We show that (1) vagal neural crest cells exhibit some developmental bias, and (2) they take separate pathways to the heart and to the gut. Together these observations suggest that prior specification dictates initial pathway choice. However, when we challenged the vagal neural crest cells with different migratory environments, we observed that the behavior of the anterior vagal neural crest cells (somite-level 1-3) exhibit considerable migratory plasticity, whereas the posterior vagal neural crest cells (somite-level 5-7) are more restricted in their behavior. We conclude that the vagal neural crest is a transitional population that has evolved between the head and the trunk.
Copyright © 2011 Wiley-Liss, Inc.
Figures





References
-
- Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development. 1997;124:3077–3087. - PubMed
-
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. - PubMed
-
- Beall AC, Rosenquist TH. Smooth muscle cells of neural crest origin form the aorticopulmonary septum in the avian embryo. Anat Rec. 1990;226:360–366. - PubMed
-
- Boot MJ, Gittenberger-De Groot AC, Van Iperen L, Hierck BP, Poelmann RE. Spatiotemporally separated cardiac neural crest subpopulations that target the outflow tract septum and pharyngeal arch arteries. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1009–1018. - PubMed
-
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol. 1986;115:44–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

