An overview of three promising mechanical, optical, and biochemical engineering approaches to improve selective photothermolysis of refractory port wine stains
- PMID: 22016324
- PMCID: PMC3281205
- DOI: 10.1007/s10439-011-0436-9
An overview of three promising mechanical, optical, and biochemical engineering approaches to improve selective photothermolysis of refractory port wine stains
Abstract
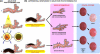
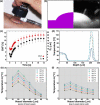
During the last three decades, several laser systems, ancillary technologies, and treatment modalities have been developed for the treatment of port wine stains (PWSs). However, approximately half of the PWS patient population responds suboptimally to laser treatment. Consequently, novel treatment modalities and therapeutic techniques/strategies are required to improve PWS treatment efficacy. This overview therefore focuses on three distinct experimental approaches for the optimization of PWS laser treatment. The approaches are addressed from the perspective of mechanical engineering (the use of local hypobaric pressure to induce vasodilation in the laser-irradiated dermal microcirculation), optical engineering (laser-speckle imaging of post-treatment flow in laser-treated PWS skin), and biochemical engineering (light- and heat-activatable liposomal drug delivery systems to enhance the extent of post-irradiation vascular occlusion).
Figures
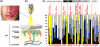
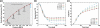







Similar articles
-
Treatment of pulsed dye laser-resistant port wine stain birthmarks.J Am Acad Dermatol. 2007 Oct;57(4):677-82. doi: 10.1016/j.jaad.2007.01.019. Epub 2007 Jul 19. J Am Acad Dermatol. 2007. PMID: 17658196 Review.
-
Port-wine stain laser treatments and novel approaches.Facial Plast Surg. 2012 Dec;28(6):611-20. doi: 10.1055/s-0032-1329936. Epub 2012 Nov 27. Facial Plast Surg. 2012. PMID: 23188689 Review.
-
An overview of clinical and experimental treatment modalities for port wine stains.J Am Acad Dermatol. 2012 Aug;67(2):289-304. doi: 10.1016/j.jaad.2011.11.938. Epub 2012 Feb 3. J Am Acad Dermatol. 2012. PMID: 22305042 Free PMC article. Review.
-
Split lesion randomized comparative study between long pulsed Nd:YAG laser 532 and 1,064 nm in treatment of facial port-wine stain.Lasers Surg Med. 2016 Nov;48(9):852-858. doi: 10.1002/lsm.22584. Epub 2016 Sep 26. Lasers Surg Med. 2016. PMID: 27669109 Clinical Trial.
-
Effects of hypobaric pressure on human skin: feasibility study for port wine stain laser therapy (part I).Lasers Surg Med. 2005 Feb;36(2):124-9. doi: 10.1002/lsm.20140. Lasers Surg Med. 2005. PMID: 15704161
Cited by
-
Quantitative assessment of graded burn wounds in a porcine model using spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI).Biomed Opt Express. 2014 Sep 8;5(10):3467-81. doi: 10.1364/BOE.5.003467. eCollection 2014 Oct 1. Biomed Opt Express. 2014. PMID: 25360365 Free PMC article.
-
Site-specific pharmaco-laser therapy: A novel treatment modality for refractory port wine stains.J Clin Transl Res. 2019 May 1;5(1):1-24. eCollection 2019 Sep 8. J Clin Transl Res. 2019. PMID: 31579838 Free PMC article. Review.
-
Histologic changes associated with talaporfin sodium-mediated photodynamic therapy in rat skin.Lasers Surg Med. 2017 Oct;49(8):767-772. doi: 10.1002/lsm.22677. Epub 2017 May 10. Lasers Surg Med. 2017. PMID: 28489260 Free PMC article.
-
Efficacy evaluation and treatment parameter optimization for laser surgery of Ota's nevus based on an advanced non-equilibrium bio-tissue heat transfer model.Lasers Med Sci. 2022 Feb;37(1):369-379. doi: 10.1007/s10103-021-03262-9. Epub 2021 Feb 8. Lasers Med Sci. 2022. PMID: 33559007
-
Utility of spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI) to non-invasively diagnose burn depth in a porcine model.Burns. 2015 Sep;41(6):1242-52. doi: 10.1016/j.burns.2015.03.001. Epub 2015 Jun 30. Burns. 2015. PMID: 26138371 Free PMC article.

