Selenoprotein K binds multiprotein complexes and is involved in the regulation of endoplasmic reticulum homeostasis
- PMID: 22016385
- PMCID: PMC3234841
- DOI: 10.1074/jbc.M111.310920
Selenoprotein K binds multiprotein complexes and is involved in the regulation of endoplasmic reticulum homeostasis
Abstract
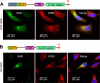
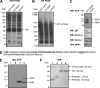
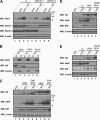
Selenoprotein K (SelK) is an 11-kDa endoplasmic reticulum (ER) protein of unknown function. Herein, we defined a new eukaryotic protein family that includes SelK, selenoprotein S (SelS), and distantly related proteins. Comparative genomics analyses indicate that this family is the most widespread eukaryotic selenoprotein family. A biochemical search for proteins that interact with SelK revealed ER-associated degradation (ERAD) components (p97 ATPase, Derlins, and SelS). In this complex, SelK showed higher affinity for Derlin-1, whereas SelS had higher affinity for Derlin-2, suggesting that these selenoproteins could determine the nature of the substrate translocated through the Derlin channel. SelK co-precipitated with soluble glycosylated ERAD substrates and was involved in their degradation. Its gene contained a functional ER stress response element, and its expression was up-regulated by conditions that induce the accumulation of misfolded proteins in the ER. Components of the oligosaccharyltransferase complex (ribophorins, OST48, and STT3A) and an ER chaperone, calnexin, were found to bind SelK. A glycosylated form of SelK was also detected, reflecting its association with the oligosaccharyltransferase complex. These data suggest that SelK is involved in the Derlin-dependent ERAD of glycosylated misfolded proteins and that the function defined by the prototypic SelK is the widespread function of selenium in eukaryotes.
Figures



References
-
- Kelleher D. J., Gilmore R. (2006) Glycobiology 16, 47R–62R - PubMed
-
- Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 - PubMed
-
- Hebert D. N., Bernasconi R., Molinari M. (2010) Semin. Cell Dev. Biol. 21, 526–532 - PubMed
-
- Bagola K., Mehnert M., Jarosch E., Sommer T. (2011) Biochim. Biophys. Acta 1808, 925–936 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

