Picomolar nitric oxide signals from central neurons recorded using ultrasensitive detector cells
- PMID: 22016390
- PMCID: PMC3234811
- DOI: 10.1074/jbc.M111.289777
Picomolar nitric oxide signals from central neurons recorded using ultrasensitive detector cells
Abstract
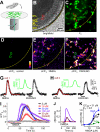
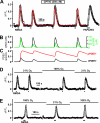
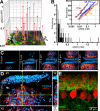
Nitric oxide (NO) is a widespread signaling molecule with potentially multifarious actions of relevance to health and disease. A fundamental determinant of how it acts is its concentration, but there remains a lack of coherent information on the patterns of NO release from its sources, such as neurons or endothelial cells, in either normal or pathological conditions. We have used detector cells having the highest recorded NO sensitivity to monitor NO release from brain tissue quantitatively and in real time. Stimulation of NMDA receptors, which are coupled to activation of neuronal NO synthase, routinely generated NO signals from neurons in cerebellar slices. The average computed peak NO concentrations varied across the anatomical layers of the cerebellum, from 12 to 130 pm. The mean value found in the hippocampus was 200 pm. Much variation in the amplitudes recorded by individual detector cells was observed, this being attributable to their location at variable distances from the NO sources. From fits to the data, the NO concentrations at the source surfaces were 120 pm to 1.4 nm, and the underlying rates of NO generation were 36-350 nm/s, depending on area. Our measurements are 4-5 orders of magnitude lower than reported by some electrode recordings in cerebellum or hippocampus. In return, they establish coherence between the NO concentrations able to elicit physiological responses in target cells through guanylyl cyclase-linked NO receptors, the concentrations that neuronal NO synthase is predicted to generate locally, and the concentrations that neurons actually produce.
Figures



Similar articles
-
Aging modulates nitric oxide synthesis and cGMP levels in hippocampus and cerebellum. Effects of amyloid beta peptide.Mol Chem Neuropathol. 1998 Aug-Dec;35(1-3):77-95. doi: 10.1007/BF02815117. Mol Chem Neuropathol. 1998. PMID: 10343972
-
Nitric oxide-evoked glutamate release and cGMP production in cerebellar slices: control by presynaptic 5-HT1D receptors.Neurochem Int. 2006 Jul;49(1):12-9. doi: 10.1016/j.neuint.2005.12.010. Epub 2006 Feb 13. Neurochem Int. 2006. PMID: 16469416
-
Age-related changes in the NMDA receptor/nitric oxide/cGMP pathway in the hippocampus and cerebellum of freely moving rats subjected to transcerebral microdialysis.Eur J Neurosci. 1995 Apr 1;7(4):694-701. doi: 10.1111/j.1460-9568.1995.tb00673.x. Eur J Neurosci. 1995. PMID: 7542528
-
Brain regional alterations in the modulation of the glutamate-nitric oxide-cGMP pathway in liver cirrhosis. Role of hyperammonemia and cell types involved.Neurochem Int. 2006 May-Jun;48(6-7):472-7. doi: 10.1016/j.neuint.2005.10.014. Epub 2006 Mar 6. Neurochem Int. 2006. PMID: 16517021 Review.
-
The NOS/sGC pathway in the rat central nervous system: a microdialysis overview.Neurochem Int. 2004 Nov;45(6):787-97. doi: 10.1016/j.neuint.2004.03.009. Neurochem Int. 2004. PMID: 15312973 Review.
Cited by
-
Hemoglobin: a nitric-oxide dioxygenase.Scientifica (Cairo). 2012;2012:683729. doi: 10.6064/2012/683729. Epub 2012 Dec 19. Scientifica (Cairo). 2012. PMID: 24278729 Free PMC article. Review.
-
Differential ligand-selective control of opposing enzymatic activities within a bifunctional c-di-GMP enzyme.Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2100657118. doi: 10.1073/pnas.2100657118. Proc Natl Acad Sci U S A. 2021. PMID: 34475207 Free PMC article.
-
How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c', Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase.Antioxid Redox Signal. 2012 Nov 1;17(9):1246-63. doi: 10.1089/ars.2012.4564. Epub 2012 Apr 10. Antioxid Redox Signal. 2012. PMID: 22356101 Free PMC article. Review.
-
Physiological activation and deactivation of soluble guanylate cyclase.Nitric Oxide. 2018 Jul 1;77:65-74. doi: 10.1016/j.niox.2018.04.011. Epub 2018 Apr 25. Nitric Oxide. 2018. PMID: 29704567 Free PMC article. Review.
-
Localised intracellular signalling in neurons.J Physiol. 2016 Jan 1;594(1):7-8. doi: 10.1113/JP271357. J Physiol. 2016. PMID: 26724480 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

