Hyaluronan synthase 2 (HAS2) promotes breast cancer cell invasion by suppression of tissue metalloproteinase inhibitor 1 (TIMP-1)
- PMID: 22016393
- PMCID: PMC3234988
- DOI: 10.1074/jbc.M111.278598
Hyaluronan synthase 2 (HAS2) promotes breast cancer cell invasion by suppression of tissue metalloproteinase inhibitor 1 (TIMP-1)
Abstract
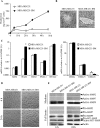
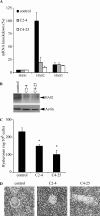
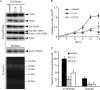
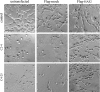
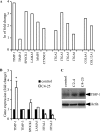
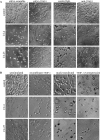
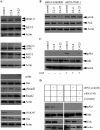
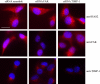
Invasion and metastasis are the primary causes of breast cancer mortality, and increased knowledge about the molecular mechanisms involved in these processes is highly desirable. High levels of hyaluronan in breast tumors have been correlated with poor patient survival. The involvement of hyaluronan in the early invasive phase of a clone of breast cancer cell line MDA-MB-231 that forms bone metastases was studied using an in vivo-like basement membrane model. The metastatic to bone tumor cells exhibited a 7-fold higher hyaluronan-synthesizing capacity compared with MDA-MB-231 cells predominately due to an increased expression of hyaluronan synthase 2 (HAS2). We found that knockdown of HAS2 completely suppressed the invasive capability of these cells by the induction of tissue metalloproteinase inhibitor 1 (TIMP-1) and dephosphorylation of focal adhesion kinase. HAS2 knockdown-mediated inhibition of basement membrane remodeling was rescued by HAS2 overexpression, transfection with TIMP-1 siRNA, or addition of TIMP-1-blocking antibodies. Moreover, knockdown of HAS2 suppressed the EGF-mediated induction of the focal adhesion kinase/PI3K/Akt signaling pathway. Thus, this study provides new insights into a possible mechanism whereby HAS2 enhances breast cancer invasion.
Figures
Similar articles
-
Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells.Int J Cancer. 2007 Jun 15;120(12):2557-67. doi: 10.1002/ijc.22550. Int J Cancer. 2007. PMID: 17315194
-
Hyaluronan synthase 2 expressed by cancer-associated fibroblasts promotes oral cancer invasion.J Exp Clin Cancer Res. 2016 Nov 25;35(1):181. doi: 10.1186/s13046-016-0458-0. J Exp Clin Cancer Res. 2016. PMID: 27884164 Free PMC article.
-
Hyaluronan synthase 2 overexpression is correlated with the tumorigenesis and metastasis of human breast cancer.Int J Clin Exp Pathol. 2015 Oct 1;8(10):12101-14. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26722395 Free PMC article.
-
Antisense-mediated suppression of hyaluronan synthase 2 inhibits the tumorigenesis and progression of breast cancer.Cancer Res. 2005 Jul 15;65(14):6139-50. doi: 10.1158/0008-5472.CAN-04-1622. Cancer Res. 2005. PMID: 16024615
-
HAS2 and CD44 in breast tumorigenesis.Adv Cancer Res. 2014;123:211-29. doi: 10.1016/B978-0-12-800092-2.00008-3. Adv Cancer Res. 2014. PMID: 25081531 Review.
Cited by
-
Over expression of hyaluronan promotes progression of HCC via CD44-mediated pyruvate kinase M2 nuclear translocation.Am J Cancer Res. 2016 Jan 15;6(2):509-21. eCollection 2016. Am J Cancer Res. 2016. PMID: 27186420 Free PMC article.
-
Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review.Int J Mol Sci. 2022 Dec 21;24(1):103. doi: 10.3390/ijms24010103. Int J Mol Sci. 2022. PMID: 36613567 Free PMC article. Review.
-
'Two-faces' of hyaluronan, a dynamic barometer of disease progression in tumor microenvironment.Discov Oncol. 2023 Jan 25;14(1):11. doi: 10.1007/s12672-023-00618-1. Discov Oncol. 2023. PMID: 36698043 Free PMC article. Review.
-
Hyaluronan: a simple polysaccharide with diverse biological functions.Acta Biomater. 2014 Apr;10(4):1558-70. doi: 10.1016/j.actbio.2013.12.019. Epub 2013 Dec 18. Acta Biomater. 2014. PMID: 24361428 Free PMC article. Review.
-
Syndecan-1 Depletion Has a Differential Impact on Hyaluronic Acid Metabolism and Tumor Cell Behavior in Luminal and Triple-Negative Breast Cancer Cells.Int J Mol Sci. 2021 May 30;22(11):5874. doi: 10.3390/ijms22115874. Int J Mol Sci. 2021. PMID: 34070901 Free PMC article.
References
-
- Kalluri R. (2003) Nat. Rev. Cancer 3, 422–433 - PubMed
-
- Bigelow R. L., Williams B. J., Carroll J. L., Daves L. K., Cardelli J. A. (2009) Breast Cancer Res. Treat. 117, 31–44 - PubMed
-
- Schrohl A. S., Holten-Andersen M. N., Peters H. A., Look M. P., Meijer-van Gelder M. E., Klijn J. G., Brünner N., Foekens J. A. (2004) Clin. Cancer Res. 10, 2289–2298 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

