MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice
- PMID: 22016411
- PMCID: PMC7968800
- DOI: 10.3174/ajnr.A2640
MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice
Abstract
MR imaging is the preferred technique for the diagnosis, treatment planning, and monitoring of patients with neoplastic CNS lesions. Conventional MR imaging, with gadolinium-based contrast enhancement, is increasingly combined with advanced, functional MR imaging techniques to offer morphologic, metabolic, and physiologic information. This article provides updated recommendations to neuroradiologists, neuro-oncologists, neurosurgeons, and radiation oncologists on the practical applications of MR imaging of neoplastic CNS lesions in adults, with particular focus on gliomas, based on a review of the clinical trial evidence and personal experiences shared at a recent international meeting of experts in neuroradiology, neuro-oncology, neurosurgery, and radio-oncology.
Figures
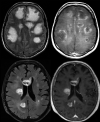
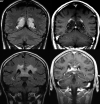
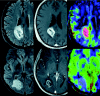
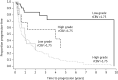
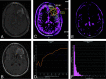
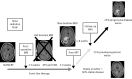
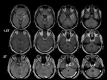
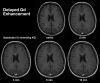

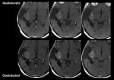
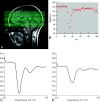
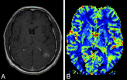
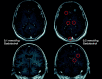
References
-
- Essig M, Weber MA, Tengg-Kobligk H, et al. . Contrast-enhanced magnetic resonance imaging of central nervous system tumors: agents, mechanisms, and applications. Top Magn Reson Imaging 2006;17:89–106 - PubMed
-
- Runge VM, Muroff LR, Wells JW. Principles of contrast enhancement in the evaluation of brain diseases: an overview. J Magn Reson Imaging 1997;7:5–13 - PubMed
-
- Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol 1999;44:275–81 - PubMed
-
- Runge VM, Muroff LR, Jinkins JR. Central nervous system: review of clinical use of contrast media. Top Magn Reson Imaging 2001;12:231–63 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
