Expression of the maize ZmGF14-6 gene in rice confers tolerance to drought stress while enhancing susceptibility to pathogen infection
- PMID: 22016430
- PMCID: PMC3254693
- DOI: 10.1093/jxb/err328
Expression of the maize ZmGF14-6 gene in rice confers tolerance to drought stress while enhancing susceptibility to pathogen infection
Abstract
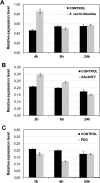
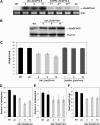
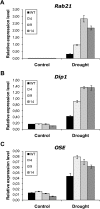
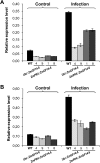
14-3-3 proteins are found in all eukaryotes where they act as regulators of diverse signalling pathways associated with a wide range of biological processes. In this study the functional characterization of the ZmGF14-6 gene encoding a maize 14-3-3 protein is reported. Gene expression analyses indicated that ZmGF14-6 is up-regulated by fungal infection and salt treatment in maize plants, whereas its expression is down-regulated by drought stress. It is reported that rice plants constitutively expressing ZmGF14-6 displayed enhanced tolerance to drought stress which was accompanied by a stronger induction of drought-associated rice genes. However, rice plants expressing ZmGF14-6 either in a constitutive or under a pathogen-inducible regime showed a higher susceptibility to infection by the fungal pathogens Fusarium verticillioides and Magnaporthe oryzae. Under infection conditions, a lower intensity in the expression of defence-related genes occurred in ZmGF14-6 rice plants. These findings support that ZmGF14-6 positively regulates drought tolerance in transgenic rice while negatively modulating the plant defence response to pathogen infection. Transient expression assays of fluorescently labelled ZmGF14-6 protein in onion epidermal cells revealed a widespread distribution of ZmGF14-6 in the cytoplasm and nucleus. Additionally, colocalization experiments of fluorescently labelled ZmGF14-6 with organelle markers, in combination with cell labelling with the endocytic tracer FM4-64, revealed a subcellular localization of ZmGF14-6 in the early endosomes. Taken together, these results improve our understanding of the role of ZmGF14-6 in stress signalling pathways, while indicating that ZmGF14-6 inversely regulates the plant response to biotic and abiotic stresses.
Figures




References
-
- Bihn EA, Paul A-L, Wang SW, Erdos GW, Ferl RJ. Localization of 14-3-3 proteins in the nuclei of Arabidopsis and maize. The Plant Journal. 1997;12:1439–1445. - PubMed
-
- Brandt J, Thordal-Christensen H, Vad K, Gregersen PL, Collinge DB. A pathogen-induced gene of barley encodes a protein showing high similarity to a protein kinase regulator. The Plant Journal. 1992;2:815–820. - PubMed
-
- Campo S, Carrascal M, Coca M, Abian J, San Segundo B. The defense response of germinating maize embryos against fungal infection: a proteomics approach. Proteomics. 2004;4:383–396. - PubMed
-
- Chen F, Li Q, Sun L, He Z. The rice 14-3-3 gene family and its involvement in responses to biotic and abiotic stress. DNA Research. 2006;13:53–63. - PubMed
-
- Chevalier D, Morris ER, Walker JC. 14-3-3 and FHA domains mediate phosphoprotein interactions. Annual Review of Plant Biology. 2009;60:67–91. - PubMed

