Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials
- PMID: 22016450
- PMCID: PMC3757919
- DOI: 10.1177/0272989X11418372
Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials
Abstract
Aim: Cost-effectiveness analyses (CEAs) may use data from cluster randomized trials (CRTs), where the unit of randomization is the cluster, not the individual. However, most studies use analytical methods that ignore clustering. This article compares alternative statistical methods for accommodating clustering in CEAs of CRTs.
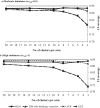
Methods: Our simulation study compared the performance of statistical methods for CEAs of CRTs with 2 treatment arms. The study considered a method that ignored clustering--seemingly unrelated regression (SUR) without a robust standard error (SE)--and 4 methods that recognized clustering--SUR and generalized estimating equations (GEEs), both with robust SE, a "2-stage" nonparametric bootstrap (TSB) with shrinkage correction, and a multilevel model (MLM). The base case assumed CRTs with moderate numbers of balanced clusters (20 per arm) and normally distributed costs. Other scenarios included CRTs with few clusters, imbalanced cluster sizes, and skewed costs. Performance was reported as bias, root mean squared error (rMSE), and confidence interval (CI) coverage for estimating incremental net benefits (INBs). We also compared the methods in a case study.
Results: Each method reported low levels of bias. Without the robust SE, SUR gave poor CI coverage (base case: 0.89 v. nominal level: 0.95). The MLM and TSB performed well in each scenario (CI coverage, 0.92-0.95). With few clusters, the GEE and SUR (with robust SE) had coverage below 0.90. In the case study, the mean INBs were similar across all methods, but ignoring clustering underestimated statistical uncertainty and the value of further research.
Conclusions: MLMs and the TSB are appropriate analytical methods for CEAs of CRTs with the characteristics described. SUR and GEE are not recommended for studies with few clusters.
Figures
References
-
- Gomes M, Grieve R, Edmunds J, Nixon R. Statistical methods for cost-effectiveness analyses that use data from cluster randomised trials: a systematic review and checklist for critical appraisal. Med Decis Making. 2011. May 24 [Epub ahead of print] - PubMed
-
- Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. London: Hodder Arnold Publishers; 2000
-
- Hayes R, Moulton L. Cluster randomised trials. Boca Raton, Florida, US: CRC Press, Taylor & Francis Group; 2009
-
- National Institute for Health and Clinical Excellence (NICE) Methods for development of NICE public health guidance. Available from URL: http://www.nice.org.uk/media/CE1/F7/CPHE_Methods_manual_LR.pdf
-
- Eckermann S, Willan AR. Expected value of information and decision making in HTA. Health Econ. 2007;16(2):195–209 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

