Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease
- PMID: 22016461
- PMCID: PMC3613145
- DOI: 10.1177/0962280211403603
Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease
Abstract
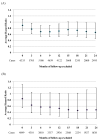
This article reviews methods for comparative effectiveness research using observational data. The basic idea is using an observational study to emulate a hypothetical randomised trial by comparing initiators versus non-initiators of treatment. After adjustment for measured baseline confounders, one can then conduct the observational analogue of an intention-to-treat analysis. We also explain two approaches to conduct the analogues of per-protocol and as-treated analyses after further adjusting for measured time-varying confounding and selection bias using inverse-probability weighting. As an example, we implemented these methods to estimate the effect of statins for primary prevention of coronary heart disease (CHD) using data from electronic medical records in the UK. Despite strong confounding by indication, our approach detected a potential benefit of statin therapy. The analogue of the intention-to-treat hazard ratio (HR) of CHD was 0.89 (0.73, 1.09) for statin initiators versus non-initiators. The HR of CHD was 0.84 (0.54, 1.30) in the per-protocol analysis and 0.79 (0.41, 1.41) in the as-treated analysis for 2 years of use versus no use. In contrast, a conventional comparison of current users versus never users of statin therapy resulted in a HR of 1.31 (1.04, 1.66). We provide a flexible and annotated SAS program to implement the proposed analyses.
Figures


References
-
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. - PubMed
-
- Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) National Institute of Heart Lung and Blood; Bethesda: 2002.
-
- Statins for the prevention of cardiovascular events. National Institute for Health and Clinical Excellence; London: 2006.
-
- Gibbons RJ, Gardner TJ, Anderson JL, Goldstein LB, Meltzer N, Weintraub WS, et al. The American Heart Association’s principles for comparative effectiveness research: a policy statement from the American Heart Association. Circulation. 2009 Jun 9;119(22):2955–62. - PubMed
-
- Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003 Nov 1;158(9):915–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

