Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial
- PMID: 22016477
- PMCID: PMC3233290
- DOI: 10.1634/theoncologist.2011-0141
Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial
Abstract
Background: Triplet chemotherapy has demonstrated manageable toxicities and a favorable response rate. The addition of cetuximab to chemotherapy can increase treatment efficacy. We evaluated the efficacy and safety of cetuximab plus 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), the ERBIRINOX regimen, as first-line treatment in patients with unresectable metastatic colorectal cancer (mCRC).
Patients and methods: In a phase II study, treatment consisted of weekly cetuximab plus biweekly. Treatment was continued for a maximum of 12 cycles and tumor response was evaluated every four cycles. The primary efficacy criterion was the complete response (CR) rate.
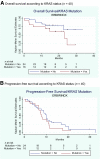
Results: From April 2006 to April 2008, 42 patients were enrolled. The median age was 60 years (range, 32-76 years). The median duration of treatment was 5.2 months (range, 0.7-8.5 months), and a median of nine cycles was given per patient (range, 1-12 cycles). Five patients (11.9%) showed a CR, with a median duration of 23.1 months (95% confidence interval [CI], 10.8-39.7 months). The objective response rate was 80.9% (95% CI, 65.9%-91.4%). The median overall and progression-free survival times were 24.7 months (95% CI, 22.6 months to not reached) and 9.5 months (95% CI, 7.6-10.4 months), respectively. The most frequent grade 3-4 adverse events were diarrhea (52%), neutropenia (38%), and asthenia (32%).
Conclusion: The ERBIRINOX regimen appears to be effective and feasible in first-line treatment of mCRC patients. These promising results led us to initiate a multicenter, randomized, phase II trial ([Research Partnership for Digestive Oncology] PRODIGE 14) in patients with potentially resectable mCRC.
Conflict of interest statement
Figures


References
-
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. - PubMed
-
- O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. - PubMed
-
- Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. - PubMed
-
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

