Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer
- PMID: 22016478
- PMCID: PMC3233284
- DOI: 10.1634/theoncologist.2011-0115
Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer
Abstract
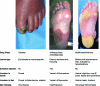
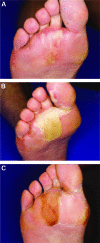
Current combination therapies for advanced breast cancer provide a modest survival benefit but with greater toxicity than with monotherapies. New combinations are needed that improve the efficacy of current treatments and have acceptable tolerability profiles. Recent clinical trials have assessed the efficacy and safety of the multikinase inhibitor sorafenib in combination with common treatments for advanced breast cancer. Sorafenib has both antiangiogenic and antiproliferative activities and is indicated for patients with unresectable hepatocellular and advanced renal cell carcinoma. Generally, sorafenib is associated with manageable, non-life-threatening adverse events. One of the more common adverse events seen with sorafenib is hand-foot skin reaction, a dermatologic toxicity usually localized to the pressure points of the palms and soles. Although hand-foot skin reaction is reversible and not life threatening, it can have a significant impact on a patient's quality of life and may necessitate dose modification. Moreover, sorafenib is being evaluated in combination with breast cancer treatments that are associated with a similar dermatologic toxicity (e.g., capecitabine-induced hand-foot syndrome). This review looks at the use of sorafenib in combination with selected chemotherapies in patients with advanced breast cancer and considers the incidence, prevention, and management of hand-foot skin reaction.
Conflict of interest statement
Figures

References
-
- Chia SK, Speers CH, D'Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. - PubMed
-
- Mauri D, Polyzos NP, Salanti G, et al. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100:1780–1791. - PubMed
-
- Joensuu H, Holli K, Heikkinen M, et al. Combination chemotherapy versus single-agent therapy as first- and second-line treatment in metastatic breast cancer: A prospective randomized trial. J Clin Oncol. 1998;16:3720–3730. - PubMed
-
- Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An intergroup trial (E1193) J Clin Oncol. 2003;21:588–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

