The metabolic regulator PGC-1α directly controls the expression of the hypothalamic neuropeptide oxytocin
- PMID: 22016516
- PMCID: PMC6623572
- DOI: 10.1523/JNEUROSCI.1798-11.2011
The metabolic regulator PGC-1α directly controls the expression of the hypothalamic neuropeptide oxytocin
Abstract
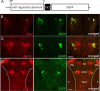
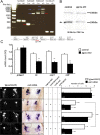
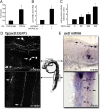
The transcriptional coactivator PGC-1α is a key regulator of cellular energy expenditure in peripheral tissues. Recent studies report that PGC-1α-null mice develop late-onset obesity and that the neuronal inactivation of PGC-1α causes increased food intake. However, the exact role of PGC-1α in the CNS remains unclear. Here we show that PGC-1α directly regulates the expression of the hypothalamic neuropeptide oxytocin, a known central regulator of appetite. We developed a unique genetic approach in the zebrafish, allowing us to monitor and manipulate PGC-1α activity in oxytocinergic neurons. We found that PGC-1α is coexpressed with oxytocin in the zebrafish hypothalamus. Targeted knockdown of the zebrafish PGC-1α gene activity caused a marked decrease in oxytocin mRNA levels and inhibited the expression of a transgenic GFP reporter driven by the oxytocin promoter. The effect of PGC-1α loss of function on oxytocin gene activity was rescued by tissue-specific re-expression of either PGC-1α or oxytocin precursor in zebrafish oxytocinergic neurons. PGC-1α activated the oxytocin promoter in a heterologous cell culture system, and overexpression of PGC-1α induced ectopic expression of oxytocin in muscles and neurons. Finally, PGC-1α forms an in vivo complex with the oxytocin promoter in fed but not fasted animals. These findings demonstrate that PGC-1α is both necessary and sufficient for the production of oxytocin, implicating hypothalamic PGC-1α in the direct activation of a hypothalamic hormone known to control energy intake.
Figures

References
-
- Adan RA, Cox JJ, Beischlag TV, Burbach JP. A composite hormone response element mediates the transactivation of the rat oxytocin gene by different classes of nuclear hormone receptors. Mol Endocrinol. 1993;7:47–57. - PubMed
-
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. - PubMed
-
- Blechman J, Borodovsky N, Eisenberg M, Nabel-Rosen H, Grimm J, Levkowitz G. Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development. 2007;134:4417–4426. - PubMed
-
- Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. - PubMed
-
- Bradford Y, Conlin T, Dunn N, Fashena D, Frazer K, Howe DG, Knight J, Mani P, Martin R, Moxon SA, Paddock H, Pich C, Ramachandran S, Ruef BJ, Ruzicka L, Bauer Schaper H, Schaper K, Shao X, Singer A, Sprague J, et al. ZFIN: enhancements and updates to the Zebrafish Model Organism Database. Nucleic Acids Res. 2011;39:D822–829. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
