Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus
- PMID: 22016518
- PMCID: PMC4126152
- DOI: 10.1523/JNEUROSCI.3922-11.2011
Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus
Abstract
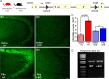
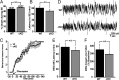
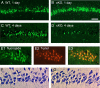
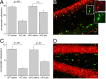
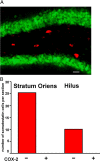
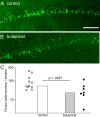
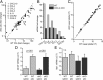
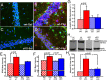
Cyclooxygenase-2 (COX-2), a source of inflammatory mediators and a multifunctional neuronal modulator, is rapidly induced in select populations of cortical neurons after status epilepticus. The consequences of rapid activity-triggered induction of COX-2 in neurons have been the subject of much study and speculation. To address this issue directly, we created a mouse in which COX-2 is conditionally ablated in selected forebrain neurons. Results following pilocarpine-induced status epilepticus indicate that neuronal COX-2 promotes early neuroprotection and then delayed neurodegeneration of CA1 pyramidal neurons, promotes neurodegeneration of nearby somatostatin interneurons in the CA1 stratum oriens and dentate hilus (which themselves do not express COX-2), intensifies a broad inflammatory reaction involving numerous cytokines and other inflammatory mediators in the hippocampus, and is essential for development of a leaky blood-brain barrier after seizures. These findings point to a profound role of seizure-induced neuronal COX-2 expression in neuropathologies that accompany epileptogenesis.
Figures

References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Abramovitz M, Adam M, Boie Y, Carrière M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
