Oligomeric-induced activity by thienyl pyrimidine compounds traps prion infectivity
- PMID: 22016521
- PMCID: PMC6623559
- DOI: 10.1523/JNEUROSCI.0547-11.2011
Oligomeric-induced activity by thienyl pyrimidine compounds traps prion infectivity
Abstract
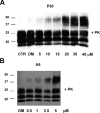
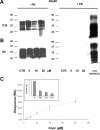
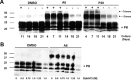
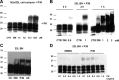
Accumulation of PrP(Sc), an abnormal form of cellular prion protein (PrP), in the brain of animals and humans leads to fatal neurodegenerative disorders known as prion diseases. Limited protease digestion of PrP(Sc) produces a truncated form called PrP(27-30) that retains prion infectivity and is the main marker of disease targeted in most diagnostic tests. In the search for new anti-prion molecules, drug-screening assays on prion-infected murine cells have been oriented toward decreasing levels of PrP(27-30). In contrast, we screened for drugs promoting multimers of PrP(27-30), illustrating a possible stabilization of mouse PrP(Sc) species, because recent studies aiming to characterize the conformational stability of various prion strains showed that stable recombinant amyloids produced more stable prion strain, leading to longest incubation time. We identified a family of thienyl pyrimidine derivatives that induce SDS-resistant dimers and trimers of PrP(27-30). Bioassays performed on mice brain homogenates treated with these compounds showed that these thienyl pyrimidine derivatives diminished prion infectivity in vivo. Oligomeric-induced activity by thienyl pyrimidine compounds is a promising approach not only to understanding the pathogenesis of prions but also for prion diagnostics. This approach could be extended to other neurodegenerative "prionopathies," such as Alzheimer's, Huntington, or Parkinson's diseases.
Figures





Similar articles
-
A Fluorescent Oligothiophene-Bis-Triazine ligand interacts with PrP fibrils and detects SDS-resistant oligomers in human prion diseases.Mol Neurodegener. 2016 Jan 26;11:11. doi: 10.1186/s13024-016-0074-7. Mol Neurodegener. 2016. PMID: 26809712 Free PMC article.
-
Low doses of bioherbicide favour prion aggregation and propagation in vivo.Sci Rep. 2018 May 23;8(1):8023. doi: 10.1038/s41598-018-25966-9. Sci Rep. 2018. PMID: 29795181 Free PMC article.
-
Thienyl pyrimidine derivatives with PrP(Sc) oligomer-inducing activity are a promising tool to study prions.Curr Top Med Chem. 2013;13(19):2477-83. doi: 10.2174/15680266113136660174. Curr Top Med Chem. 2013. PMID: 24059332 Review.
-
Development of oligomeric prion-protein aggregates in a mouse model of prion disease.J Pathol. 2009 Sep;219(1):123-30. doi: 10.1002/path.2576. J Pathol. 2009. PMID: 19479969
-
[Mechanisms of prion transmission].Nihon Rinsho. 2007 Aug;65(8):1391-5. Nihon Rinsho. 2007. PMID: 17695274 Review. Japanese.
Cited by
-
Therapeutic strategies for identifying small molecules against prion diseases.Cell Tissue Res. 2023 Apr;392(1):337-347. doi: 10.1007/s00441-021-03573-x. Epub 2022 Jan 6. Cell Tissue Res. 2023. PMID: 34989851 Review.
-
Comparing the energy landscapes for native folding and aggregation of PrP.Prion. 2016 May 3;10(3):207-20. doi: 10.1080/19336896.2016.1173297. Prion. 2016. PMID: 27191683 Free PMC article.
-
A Fluorescent Oligothiophene-Bis-Triazine ligand interacts with PrP fibrils and detects SDS-resistant oligomers in human prion diseases.Mol Neurodegener. 2016 Jan 26;11:11. doi: 10.1186/s13024-016-0074-7. Mol Neurodegener. 2016. PMID: 26809712 Free PMC article.
-
Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross-seeding behavior.Protein Sci. 2018 Nov;27(11):1901-1909. doi: 10.1002/pro.3499. Epub 2018 Oct 19. Protein Sci. 2018. PMID: 30125425 Free PMC article.
-
Low doses of bioherbicide favour prion aggregation and propagation in vivo.Sci Rep. 2018 May 23;8(1):8023. doi: 10.1038/s41598-018-25966-9. Sci Rep. 2018. PMID: 29795181 Free PMC article.
References
-
- Adjou KT, Demaimay R, Deslys JP, Lasmézas CI, Beringue V, Demart S, Lamoury F, Seman M, Dormont D. MS-8209, a water-soluble amphotericin B derivative, affects both scrapie agent replication and PrPres accumulation in Syrian hamster scrapie. J Gen Virol. 1999;80:1079–1085. - PubMed
-
- Andreoletti O. Basel: Birkhäuser Basel; 2004. Techniques in prion research (methods and tools in biosciences and medicine) pp. 82–96.
-
- Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–317. - PubMed
-
- Beringue V, Adjou KT, Lamoury F, Maignien T, Deslys JP, Race R, Dormont D. Opposite effects of dextran sulfate 500, the polyene antibiotic MS-8209, and Congo red on accumulation of the protease-resistant isoform of PrP in the spleens of mice inoculated intraperitoneally with the scrapie agent. J Virol. 2000;74:5432–5440. - PMC - PubMed
-
- Bocharova OV, Breydo L, Parfenov AS, Salnikov VV, Baskakov IV. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrP(Sc) J Mol Biol. 2005;346:645–659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
