Newborn neurons in the olfactory bulb selected for long-term survival through olfactory learning are prematurely suppressed when the olfactory memory is erased
- PMID: 22016522
- PMCID: PMC6623563
- DOI: 10.1523/JNEUROSCI.3677-11.2011
Newborn neurons in the olfactory bulb selected for long-term survival through olfactory learning are prematurely suppressed when the olfactory memory is erased
Abstract
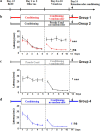
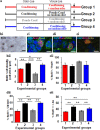
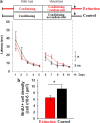
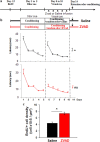
A role for newborn neurons in olfactory memory has been proposed based on learning-dependent modulation of olfactory bulb neurogenesis in adults. We hypothesized that if newborn neurons support memory, then they should be suppressed by memory erasure. Using an ecological approach in mice, we showed that behaviorally breaking a previously learned odor-reward association prematurely suppressed newborn neurons selected to survive during initial learning. Furthermore, intrabulbar infusions of the caspase pan-inhibitor ZVAD (benzyloxycarbonyl-Val-Ala-Asp) during the behavioral odor-reward extinction prevented newborn neurons death and erasure of the odor-reward association. Newborn neurons thus contribute to the bulbar network plasticity underlying long-term memory.
Figures
Similar articles
-
Involvement of newborn neurons in olfactory associative learning? The operant or non-operant component of the task makes all the difference.J Neurosci. 2011 Aug 31;31(35):12455-60. doi: 10.1523/JNEUROSCI.2919-11.2011. J Neurosci. 2011. PMID: 21880907 Free PMC article.
-
Neurogenic correlates of an olfactory discrimination task in the adult olfactory bulb.Eur J Neurosci. 2006 Dec;24(12):3578-88. doi: 10.1111/j.1460-9568.2006.05235.x. Eur J Neurosci. 2006. PMID: 17229106
-
Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory.J Neurosci. 2002 Apr 1;22(7):2679-89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. J Neurosci. 2002. PMID: 11923433 Free PMC article.
-
Newborn neurons in the adult olfactory bulb: unique properties for specific odor behavior.Behav Brain Res. 2012 Feb 14;227(2):480-9. doi: 10.1016/j.bbr.2011.08.001. Epub 2011 Aug 6. Behav Brain Res. 2012. PMID: 21843557 Review.
-
Is adult neurogenesis essential for olfaction?Trends Neurosci. 2011 Jan;34(1):20-30. doi: 10.1016/j.tins.2010.09.006. Epub 2010 Oct 25. Trends Neurosci. 2011. PMID: 20980064 Review.
Cited by
-
Microglial activation - tuning and pruning adult neurogenesis.Front Pharmacol. 2012 Mar 9;3:41. doi: 10.3389/fphar.2012.00041. eCollection 2012. Front Pharmacol. 2012. PMID: 22408626 Free PMC article.
-
Adult Olfactory Bulb Neurogenesis.Cold Spring Harb Perspect Biol. 2016 Aug 1;8(8):a018945. doi: 10.1101/cshperspect.a018945. Cold Spring Harb Perspect Biol. 2016. PMID: 27235474 Free PMC article. Review.
-
Age-dependent regional changes in the rostral migratory stream.Neurobiol Aging. 2013 Jul;34(7):1873-81. doi: 10.1016/j.neurobiolaging.2013.01.015. Epub 2013 Feb 15. Neurobiol Aging. 2013. PMID: 23419702 Free PMC article.
-
The functional significance of newly born neurons integrated into olfactory bulb circuits.Front Neurosci. 2014 May 26;8:121. doi: 10.3389/fnins.2014.00121. eCollection 2014. Front Neurosci. 2014. PMID: 24904263 Free PMC article. Review.
-
The Role of Astrocytes in the Generation, Migration, and Integration of New Neurons in the Adult Olfactory Bulb.Front Neurosci. 2016 Apr 5;10:149. doi: 10.3389/fnins.2016.00149. eCollection 2016. Front Neurosci. 2016. PMID: 27092050 Free PMC article. Review.
References
-
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the olfactory bulb. Nat Neurosci. 2003;6:507–518. - PubMed
-
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
