Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse
- PMID: 22016524
- PMCID: PMC3213690
- DOI: 10.1523/JNEUROSCI.3590-11.2011
Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse
Abstract
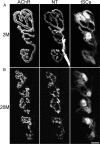
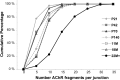
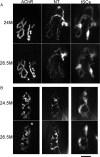
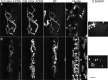
Vertebrate neuromuscular junctions are highly stable synapses, retaining the morphology they achieve in early postnatal development throughout most of life. However, these synapses undergo dramatic change during aging. The acetylcholine receptors (AChRs) change from smooth gutters into fragmented islands, and the nerve terminals change similarly to be varicosities apposed to these islands. These changes have been attributed to a slow deterioration in mechanisms maintaining the synapse. We have used repeated, vital imaging to investigate how these changes occur in the sternomastoid muscle of aging mice. We have found, contrary to expectation, that individual junctions change infrequently, but change, when it occurs, is sudden and dramatic. The change mimics that reported previously for cases in which muscle fibers are deliberately damaged: most of the AChRs present disappear rapidly and are replaced by a new set of receptors that become fragmented. The fiber segment underneath the synapse has centrally located nuclei, showing that this segment has undergone necrosis, quickly regenerated, and been reinnervated with an altered synapse. We show that necrotic events are common in aged muscle and have likely been missed previously as a cause of the alterations in aging because central nuclei are a transient phenomenon and the necrotic events at the junction infrequent. However, the changes are permanent and accumulate over time. Interventions to reduce the neuromuscular changes during aging should likely focus on making muscle fibers resistant to injury.
Figures


Similar articles
-
Nerve terminal growth remodels neuromuscular synapses in mice following regeneration of the postsynaptic muscle fiber.J Neurosci. 2011 Sep 14;31(37):13191-203. doi: 10.1523/JNEUROSCI.2953-11.2011. J Neurosci. 2011. PMID: 21917802 Free PMC article.
-
In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions.J Neurosci. 1993 Feb;13(2):834-55. doi: 10.1523/JNEUROSCI.13-02-00834.1993. J Neurosci. 1993. PMID: 8426240 Free PMC article.
-
Src-family kinases stabilize the neuromuscular synapse in vivo via protein interactions, phosphorylation, and cytoskeletal linkage of acetylcholine receptors.J Neurosci. 2005 Nov 9;25(45):10479-93. doi: 10.1523/JNEUROSCI.2103-05.2005. J Neurosci. 2005. PMID: 16280586 Free PMC article.
-
Interactions between nerve and muscle: synapse elimination at the developing neuromuscular junction.Dev Biol. 1993 Mar;156(1):1-10. doi: 10.1006/dbio.1993.1054. Dev Biol. 1993. PMID: 8449362 Review.
-
Neurophysiology of the neuromuscular junction: overview.Ann N Y Acad Sci. 2003 Sep;998:1-10. Ann N Y Acad Sci. 2003. PMID: 14592857 Review.
Cited by
-
Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool.Hum Mol Genet. 2013 Oct 1;22(19):3976-86. doi: 10.1093/hmg/ddt251. Epub 2013 Jun 10. Hum Mol Genet. 2013. PMID: 23760083 Free PMC article.
-
Functional and Structural Changes in Diaphragm Neuromuscular Junctions in Early Aging.Int J Mol Sci. 2024 Aug 17;25(16):8959. doi: 10.3390/ijms25168959. Int J Mol Sci. 2024. PMID: 39201644 Free PMC article.
-
Overexpression of PGC-1α in aging muscle enhances a subset of young-like molecular patterns.Aging Cell. 2018 Apr;17(2):e12707. doi: 10.1111/acel.12707. Epub 2018 Feb 10. Aging Cell. 2018. PMID: 29427317 Free PMC article.
-
Matrisome, innervation and oxidative metabolism affected in older compared with younger males with similar physical activity.J Cachexia Sarcopenia Muscle. 2021 Oct;12(5):1214-1231. doi: 10.1002/jcsm.12753. Epub 2021 Jul 4. J Cachexia Sarcopenia Muscle. 2021. PMID: 34219410 Free PMC article.
-
Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice.Front Physiol. 2015 Sep 9;6:252. doi: 10.3389/fphys.2015.00252. eCollection 2015. Front Physiol. 2015. PMID: 26441672 Free PMC article.
References
-
- Akaaboune M, Culican SM, Turney SG, Lichtman JW. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science. 1999;286:503–507. - PubMed
-
- Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve Suppl. 1997;5:S83–S87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
