Dopaminergic modulation of the striatal microcircuit: receptor-specific configuration of cell assemblies
- PMID: 22016530
- PMCID: PMC6623553
- DOI: 10.1523/JNEUROSCI.3226-11.2011
Dopaminergic modulation of the striatal microcircuit: receptor-specific configuration of cell assemblies
Abstract
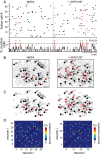
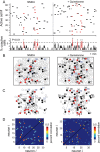
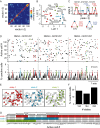
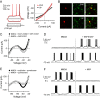
Selection and inhibition of motor behaviors are related to the coordinated activity and compositional capabilities of striatal cell assemblies. Striatal network activity represents a main step in basal ganglia processing. The dopaminergic system differentially regulates distinct populations of striatal medium spiny neurons (MSNs) through the activation of D(1)- or D(2)-type receptors. Although postsynaptic and presynaptic actions of these receptors are clearly different in MSNs during cell-focused studies, their activation during network activity has shown inconsistent responses. Therefore, using electrophysiological techniques, functional multicell calcium imaging, and neuronal population analysis in rat corticostriatal slices, we describe the effect of selective dopaminergic receptor activation in the striatal network by observing cell assembly configurations. At the microcircuit level, during striatal network activity, the selective activation of either D(1)- or D(2)-type receptors is reflected as overall increases in neuronal synchronization. However, graph theory techniques applied to the transitions between network states revealed receptor-specific configurations of striatal cell assemblies: D(1) receptor activation generated closed trajectories with high recurrence and few alternate routes favoring the selection of specific sequences, whereas D(2) receptor activation created trajectories with low recurrence and more alternate pathways while promoting diverse transitions among neuronal pools. At the single-cell level, the activation of dopaminergic receptors enhanced the negative-slope conductance region (NSCR) in D(1)-type-responsive cells, whereas in neurons expressing D(2)-type receptors, the NSCR was decreased. Consequently, receptor-specific network dynamics most probably result from the interplay of postsynaptic and presynaptic dopaminergic actions.
Figures


Similar articles
-
Activation of the cholinergic system endows compositional properties to striatal cell assemblies.J Neurophysiol. 2009 Feb;101(2):737-49. doi: 10.1152/jn.90975.2008. Epub 2008 Nov 19. J Neurophysiol. 2009. PMID: 19019973
-
Encoding network states by striatal cell assemblies.J Neurophysiol. 2008 Mar;99(3):1435-50. doi: 10.1152/jn.01131.2007. Epub 2008 Jan 9. J Neurophysiol. 2008. PMID: 18184883
-
Dopaminergic modulation of excitatory postsynaptic currents in rat neostriatal neurons.J Neurophysiol. 1997 Sep;78(3):1248-55. doi: 10.1152/jn.1997.78.3.1248. J Neurophysiol. 1997. PMID: 9310416
-
Modeling influences of dopamine on synchronization behavior of striatum.Network. 2017;28(1):28-52. doi: 10.1080/0954898X.2017.1378824. Epub 2017 Oct 6. Network. 2017. PMID: 28985088 Review.
-
Dopamine-modulated dynamic cell assemblies generated by the GABAergic striatal microcircuit.Neural Netw. 2009 Oct;22(8):1174-88. doi: 10.1016/j.neunet.2009.07.018. Epub 2009 Jul 19. Neural Netw. 2009. PMID: 19646846 Review.
Cited by
-
Paradoxical Boosting of Weak and Strong Spatial Memories by Hippocampal Dopamine Uncaging.eNeuro. 2024 May 29;11(5):ENEURO.0469-23.2024. doi: 10.1523/ENEURO.0469-23.2024. Print 2024 May. eNeuro. 2024. PMID: 38755011 Free PMC article.
-
Global actions of nicotine on the striatal microcircuit.Front Syst Neurosci. 2013 Nov 6;7:78. doi: 10.3389/fnsys.2013.00078. eCollection 2013. Front Syst Neurosci. 2013. PMID: 24223538 Free PMC article.
-
Exploring the role of striatal D1 and D2 medium spiny neurons in action selection using a virtual robotic framework.Eur J Neurosci. 2019 Mar;49(6):737-753. doi: 10.1111/ejn.14021. Epub 2018 Aug 1. Eur J Neurosci. 2019. PMID: 29917291 Free PMC article.
-
Nicotine Increases Spontaneous Glutamate Release in the Rostromedial Tegmental Nucleus.Front Neurosci. 2021 Jan 13;14:604583. doi: 10.3389/fnins.2020.604583. eCollection 2020. Front Neurosci. 2021. PMID: 33519359 Free PMC article.
-
The balance of striatal feedback transmission is disrupted in a model of parkinsonism.J Neurosci. 2013 Mar 13;33(11):4964-75. doi: 10.1523/JNEUROSCI.4721-12.2013. J Neurosci. 2013. PMID: 23486967 Free PMC article.
References
-
- Abeles M, Hayon G, Lehmann D. Modeling compositionality by dynamic binding of synfire chains. J Comput Neurosci. 2004;17:179–201. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
