Properties of slow oscillation during slow-wave sleep and anesthesia in cats
- PMID: 22016533
- PMCID: PMC3209581
- DOI: 10.1523/JNEUROSCI.2339-11.2011
Properties of slow oscillation during slow-wave sleep and anesthesia in cats
Abstract
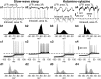
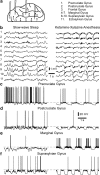
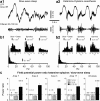
Deep anesthesia is commonly used as a model of slow-wave sleep (SWS). Ketamine-xylazine anesthesia reproduces the main features of sleep slow oscillation: slow, large-amplitude waves in field potential, which are generated by the alternation of hyperpolarized and depolarized states of cortical neurons. However, direct quantitative comparison of field potential and membrane potential fluctuations during natural sleep and anesthesia is lacking, so it remains unclear how well the properties of sleep slow oscillation are reproduced by the ketamine-xylazine anesthesia model. Here, we used field potential and intracellular recordings in different cortical areas in the cat to directly compare properties of slow oscillation during natural sleep and ketamine-xylazine anesthesia. During SWS cortical activity showed higher power in the slow/delta (0.1-4 Hz) and spindle (8-14 Hz) frequency range, whereas under anesthesia the power in the gamma band (30-100 Hz) was higher. During anesthesia, slow waves were more rhythmic and more synchronous across the cortex. Intracellular recordings revealed that silent states were longer and the amplitude of membrane potential around transition between active and silent states was bigger under anesthesia. Slow waves were mostly uniform across cortical areas under anesthesia, but in SWS, they were most pronounced in associative and visual areas but smaller and less regular in somatosensory and motor cortices. We conclude that, although the main features of the slow oscillation in sleep and anesthesia appear similar, multiple cellular and network features are differently expressed during natural SWS compared with ketamine-xylazine anesthesia.
Figures




References
-
- Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–s693. - PubMed
-
- Bignall KE. Comparison of optic afferents to primary visual and polysensory areas of cat neocortex. Exp Neurol. 1967;17:327–343. - PubMed
-
- Blake H, Gerard RW. Brain potentials during sleep. Am J Physiol. 1937;119:692–703.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
