Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea
- PMID: 22016543
- PMCID: PMC3213862
- DOI: 10.1523/JNEUROSCI.2743-11.2011
Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea
Abstract
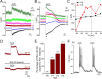
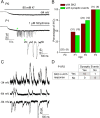
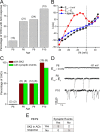
In the developing mammalian cochlea, the sensory hair cells receive efferent innervation originating in the superior olivary complex. This input is mediated by α9/α10 nicotinic acetylcholine receptors (nAChRs) and is inhibitory due to the subsequent activation of calcium-dependent SK2 potassium channels. We examined the acquisition of this cholinergic efferent input using whole-cell voltage-clamp recordings from inner hair cells (IHCs) in acutely excised apical turns of the rat cochlea from embryonic day 21 to postnatal day 8 (P8). Responses to 1 mm acetylcholine (ACh) were detected from P0 on in almost every IHC. The ACh-activated current amplitude increased with age and demonstrated the same pharmacology as α9-containing nAChRs. Interestingly, at P0, the ACh response was not coupled to SK2 channels, so that the initial cholinergic response was excitatory and could trigger action potentials in IHCs. Coupling to SK current was detected earliest at P1 in a subset of IHCs and by P3 in every IHC studied. Clustered nAChRs and SK2 channels were found on IHCs from P1 on using Alexa Fluor 488 conjugated α-bungarotoxin and SK2 immunohistochemistry. The number of nAChRs clusters increased with age to 16 per IHC at P8. Cholinergic efferent synaptic currents first appeared in a subset of IHCs at P1 and by P3 in every IHC studied, contemporaneously with ACh-evoked SK currents, suggesting that SK2 channels may be necessary at onset of synaptic function. An analogous pattern of development was observed for the efferent synapses that form later (P6-P8) on outer hair cells in the basal cochlea.
Figures


References
-
- Anand R, Peng X, Lindstrom J. Homomeric and native alpha 7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett. 1993;327:241–246. - PubMed
-
- Bruce LL, Kingsley J, Nichols DH, Fritzsch B. The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci. 1997;15:671–692. - PubMed
-
- Bruce LL, Christensen MA, Warr WB. Postnatal development of efferent synapses in the rat cochlea. J Comp Neurol. 2000;423:532–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DC006476/DC/NIDCD NIH HHS/United States
- R01 DC006476/DC/NIDCD NIH HHS/United States
- R01 DC000276/DC/NIDCD NIH HHS/United States
- P01 GM048677/GM/NIGMS NIH HHS/United States
- R01DC000276/DC/NIDCD NIH HHS/United States
- MH53631/MH/NIMH NIH HHS/United States
- R29 MH053631/MH/NIMH NIH HHS/United States
- P30 DC005211/DC/NIDCD NIH HHS/United States
- R24DK064388/DK/NIDDK NIH HHS/United States
- R24 DK064388/DK/NIDDK NIH HHS/United States
- R01 DC001508/DC/NIDCD NIH HHS/United States
- GM48677/GM/NIGMS NIH HHS/United States
- R01 MH053631/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
