Posttraining ablation of adult-generated neurons degrades previously acquired memories
- PMID: 22016545
- PMCID: PMC6623574
- DOI: 10.1523/JNEUROSCI.3432-11.2011
Posttraining ablation of adult-generated neurons degrades previously acquired memories
Abstract
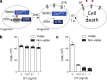
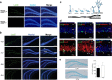
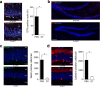
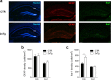
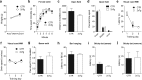
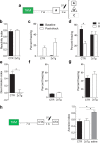
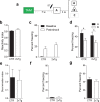
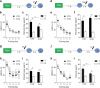
New neurons are continuously generated in the subgranular zone of the adult hippocampus and, once sufficiently mature, are thought to integrate into hippocampal memory circuits. However, whether they play an essential role in subsequent memory expression is not known. Previous studies have shown that suppression of adult neurogenesis often (but not always) impairs subsequent hippocampus-dependent learning (i.e., produces anterograde effects). A major challenge for these studies is that these new neurons represent only a small subpopulation of all dentate granule cells, and so there is large potential for either partial or complete compensation by granule cells generated earlier on during development. A potentially more powerful approach to investigate this question would be to ablate adult-generated neurons after they have already become part of a memory trace (i.e., retrograde effects). Here we developed a diphtheria toxin-based strategy in mice that allowed us to selectively ablate a population of predominantly mature, adult-generated neurons either before or after learning, without affecting ongoing neurogenesis. Removal of these neurons before learning did not prevent the formation of new contextual fear or water maze memories. In contrast, removal of an equivalent population after learning degraded existing contextual fear and water maze memories, without affecting nonhippocampal memory. Ablation of these adult-generated neurons even 1 month after learning produced equivalent memory degradation in the water maze. These retrograde effects suggest that adult-generated neurons form a critical and enduring component of hippocampal memory traces.
Figures


References
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. - PubMed
-
- Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–235. - PubMed
-
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. - PubMed
-
- Chen GH, Wang YJ, Zhang LQ, Zhou JN. Age- and sex-related disturbance in a battery of sensorimotor and cognitive tasks in Kunming mice. Physiol Behav. 2004;83:531–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
