Intact bilateral resting-state networks in the absence of the corpus callosum
- PMID: 22016549
- PMCID: PMC3221732
- DOI: 10.1523/JNEUROSCI.1453-11.2011
Intact bilateral resting-state networks in the absence of the corpus callosum
Abstract
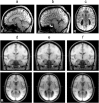
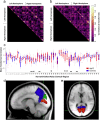
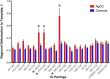
Temporal correlations between different brain regions in the resting-state BOLD signal are thought to reflect intrinsic functional brain connectivity (Biswal et al., 1995; Greicius et al., 2003; Fox et al., 2007). The functional networks identified are typically bilaterally distributed across the cerebral hemispheres, show similarity to known white matter connections (Greicius et al., 2009), and are seen even in anesthetized monkeys (Vincent et al., 2007). Yet it remains unclear how they arise. Here we tested two distinct possibilities: (1) functional networks arise largely from structural connectivity constraints, and generally require direct interactions between functionally coupled regions mediated by white-matter tracts; and (2) functional networks emerge flexibly with the development of normal cognition and behavior and can be realized in multiple structural architectures. We conducted resting-state fMRI in eight adult humans with complete agenesis of the corpus callosum (AgCC) and normal intelligence, and compared their data to those from eight healthy matched controls. We performed three main analyses: anatomical region-of-interest-based correlations to test homotopic functional connectivity, independent component analysis (ICA) to reveal functional networks with a data-driven approach, and ICA-based interhemispheric correlation analysis. Both groups showed equivalently strong homotopic BOLD correlation. Surprisingly, almost all of the group-level independent components identified in controls were observed in AgCC and were predominantly bilaterally symmetric. The results argue that a normal complement of resting-state networks and intact functional coupling between the hemispheres can emerge in the absence of the corpus callosum, favoring the second over the first possibility listed above.
Figures



References
-
- Andersson JL, Smith S, Jenkinson M. FNIRT: FMRIB non-linear image registration tool. Paper presented at Meeting for the Organization for Human Brain Mapping; June; Melbourne, Australia. 2008.
-
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. - PubMed
-
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
