Phospholipase C and protein kinase C-β 2 mediate insulin-like growth factor II-dependent sphingosine kinase 1 activation
- PMID: 22016563
- PMCID: PMC3231827
- DOI: 10.1210/me.2011-0101
Phospholipase C and protein kinase C-β 2 mediate insulin-like growth factor II-dependent sphingosine kinase 1 activation
Abstract
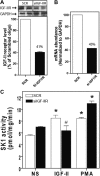
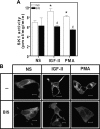
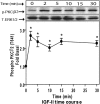
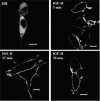
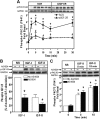
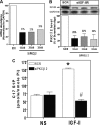
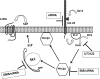
We recently reported that IGF-II binding to the IGF-II/mannose-6-phosphate (M6P) receptor activates the ERK1/2 cascade by triggering sphingosine kinase 1 (SK1)-dependent transactivation of G protein-coupled sphingosine 1-phosphate (S1P) receptors. Here, we investigated the mechanism of IGF-II/M6P receptor-dependent sphingosine kinase 1 (SK1) activation in human embryonic kidney 293 cells. Pretreating cells with protein kinase C (PKC) inhibitor, bisindolylmaleimide-I, abolished IGF-II-stimulated translocation of green fluorescent protein (GFP)-tagged SK1 to the plasma membrane and activation of endogenous SK1, implicating PKC as an upstream regulator of SK1. Using confocal microscopy to examine membrane translocation of GFP-tagged PKCα, β1, β2, δ, and ζ, we found that IGF-II induced rapid, transient, and isoform-specific translocation of GFP-PKCβ2 to the plasma membrane. Immunoblotting of endogenous PKC phosphorylation confirmed PKCβ2 activation in response to IGF-II. Similarly, IGF-II stimulation caused persistent membrane translocation of the kinase-deficient GFP-PKCβ2 (K371R) mutant, which does not dissociate from the membrane after translocation. IGF-II stimulation increased diacylglycerol (DAG) levels, the established activator of classical PKC. Interestingly, the polyunsaturated fraction of DAG was increased, indicating involvement of phosphatidyl inositol/phospholipase C (PLC). Pretreating cells with the PLC inhibitor, U73122, attenuated IGF-II-dependent DAG production and PKCβ2 phosphorylation, blocked membrane translocation of the kinase-deficient GFP-PKCβ2 (K371R) mutant, and reduced sphingosine 1-phosphate production, suggesting that PLC/PKCβ2 are upstream regulators of SK1 in the pathway. Taken together, these data provide evidence that activation of PLC and PKCβ2 by the IGF-II/M6P receptor are required for the activation of SK1.
Figures

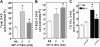



References
-
- Hawkes C, Amritraj A, Macdonald RG, Jhamandas JH, Kar S. 2007. Heterotrimeric G proteins and the single-transmembrane domain IGF-II/M6P receptor: functional interaction and relevance to cell signaling. Mol Neurobiol 35:329–345 - PubMed
-
- Matsunaga H, Nishimoto I, Kojima I, Yamashita N, Kurokawa K, Ogata E. 1988. Activation of a calcium-permeable cation channel by insulin-like growth factor II in BALB/c 3T3 cells. Am J Physiol 255:C442–C446 - PubMed
-
- Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, Huang CY. 2008. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gαq interaction and protein kinase C-α/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol 197:381–390 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

