Nuclear factor E2-related factor 2 dependent overexpression of sulfiredoxin and peroxiredoxin III in human lung cancer
- PMID: 22016591
- PMCID: PMC3192203
- DOI: 10.3904/kjim.2011.26.3.304
Nuclear factor E2-related factor 2 dependent overexpression of sulfiredoxin and peroxiredoxin III in human lung cancer
Abstract
Background/aims: Oxidative stress results in protein oxidation and is implicated in carcinogenesis. Sulfiredoxin (Srx) is responsible for the enzymatic reversal of inactivated peroxiredoxin (Prx). Nuclear factor E2-related factor 2 (Nrf2) binds to antioxidant responsive elements and upregulates the expression of Srx and Prx during oxidative stress. We aimed to elucidate the biological functions and potential roles of Srx in lung cancer.
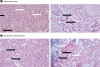
Methods: To study the roles of Srx and Prx III in lung cancer, we compared the protein levels of Nrf2, Prxs, thioredoxin, and Srx in 40 surgically resected human lung cancer tissues using immunoblot and immunohistochemical analyses. Transforming growth factor-β(1), tumor necrosis factor-α, and camptothecin treatment were used to examine Prx III inactivation in Mv1Lu mink lung epithelial cells and A549 lung cancer cells.
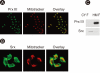
Results: Prx I and Prx III proteins were markedly overexpressed in lung cancer tissues. A significant increase in the oxidized form of a cysteine sulfhydryl at the catalytic site of Prxs was found in carcinogenic lung tissue compared to normal lung tissue. Densitometric analyses of immunoblot data revealed significant Srx expression, which was higher in squamous cell carcinoma tissue (60%, 12/20) than in adenocarcinoma (20%, 4/20). Also, Nrf2 was present in the nuclear compartment of cancer cells.
Conclusions: Srx and Prx III proteins were markedly overexpressed in human squamous cell carcinoma, suggesting that these proteins may play a protective role against oxidative injury and compensate for the high rate of mitochondrial metabolism in lung cancer.
Keywords: GA-binding protein transcription factor; Lung neoplasms; Peroxiredoxins; Sulfiredoxin.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures








Similar articles
-
Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia.Antioxid Redox Signal. 2009 May;11(5):937-48. doi: 10.1089/ars.2008.2325. Antioxid Redox Signal. 2009. PMID: 19086807
-
Sulfiredoxin-Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7004-9. doi: 10.1073/pnas.1013012108. Epub 2011 Apr 12. Proc Natl Acad Sci U S A. 2011. PMID: 21487000 Free PMC article.
-
Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver.Hepatology. 2011 Mar;53(3):945-53. doi: 10.1002/hep.24104. Epub 2011 Feb 11. Hepatology. 2011. PMID: 21319188
-
Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression.Free Radic Biol Med. 2012 Aug 1;53(3):447-56. doi: 10.1016/j.freeradbiomed.2012.05.020. Epub 2012 May 24. Free Radic Biol Med. 2012. PMID: 22634055 Review.
-
The sulfiredoxin-peroxiredoxin (Srx-Prx) axis in cell signal transduction and cancer development.Cancer Lett. 2015 Oct 1;366(2):150-9. doi: 10.1016/j.canlet.2015.07.002. Epub 2015 Jul 10. Cancer Lett. 2015. PMID: 26170166 Free PMC article. Review.
Cited by
-
Anti-cancer effect of snake venom toxin through down regulation of AP-1 mediated PRDX6 expression.Oncotarget. 2015 Sep 8;6(26):22139-51. doi: 10.18632/oncotarget.4192. Oncotarget. 2015. PMID: 26061816 Free PMC article.
-
Sulfiredoxin-1 is a promising novel prognostic biomarker for hepatocellular carcinoma.Cancer Med. 2020 Nov;9(22):8318-8332. doi: 10.1002/cam4.3430. Epub 2020 Sep 21. Cancer Med. 2020. PMID: 32955798 Free PMC article.
-
Integrative analysis the characterization of peroxiredoxins in pan-cancer.Cancer Cell Int. 2021 Jul 10;21(1):366. doi: 10.1186/s12935-021-02064-x. Cancer Cell Int. 2021. PMID: 34246267 Free PMC article.
-
Hydrogen peroxide - production, fate and role in redox signaling of tumor cells.Cell Commun Signal. 2015 Sep 14;13:39. doi: 10.1186/s12964-015-0118-6. Cell Commun Signal. 2015. PMID: 26369938 Free PMC article. Review.
-
Mutation spectrum of hepatocellular carcinoma from eastern-European patients betrays the impact of a complex exposome.J Expo Sci Environ Epidemiol. 2015 May;25(3):256-63. doi: 10.1038/jes.2014.16. Epub 2014 Apr 16. J Expo Sci Environ Epidemiol. 2015. PMID: 24736102
References
-
- Rhee SG. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. - PubMed
-
- Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. - PubMed
-
- Chang TS, Jeong W, Woo HA, Lee SM, Park S, Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
