Pluripotent stem cells for the study of CNS development
- PMID: 22016722
- PMCID: PMC3191505
- DOI: 10.3389/fnmol.2011.00030
Pluripotent stem cells for the study of CNS development
Abstract
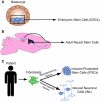
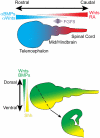
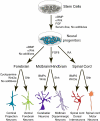
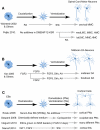
The mammalian central nervous system is a complex neuronal network consisting of a diverse array of cellular subtypes generated in a precise spatial and temporal pattern throughout development. Achieving a greater understanding of the molecular and genetic mechanisms that direct a relatively uniform population of neuroepithelial progenitors into diverse neuronal subtypes remains a significant challenge. The advent of pluripotent stem cell (PSC) technology allows researchers to generate diverse neural populations in vitro. Although the primary focus of PSC-derived neural cells has been their therapeutic potential, utilizing PSCs to study neurodevelopment is another frequently overlooked and equally important application. In this review, we explore the potential for utilizing PSCs to study neural development. We introduce the types of neurodevelopmental questions that PSCs can help to address, and we discuss the different strategies and technologies that researchers use to generate diverse subtypes of PSC-derived neurons. Additionally, we highlight the derivation of several thoroughly characterized neural subtypes; spinal motoneurons, midbrain dopaminergic neurons and cortical neurons. We hope that this review encourages researchers to develop innovative strategies for using PSCs for the study of mammalian, and specifically human, neurodevelopment.
Keywords: derivation; development; embryonic; nervous system; neurons; pluripotent; stem cells.
Figures
References
-
- Boulting G. L., Kiskinis E., Croft G. F., Amoroso M. W., Oakley D. H., Wainger B. J., Williams D. J., Kahler D. J., Yamaki M., Davidow L., Rodolfa C. T., Dimos J. T., Mikkilineni S., Macdermott A. B., Woolf C. J., Henderson C. E., Wichterle H., Eggan K. (2011). A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 29, 279–28610.1038/nbt.1783 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

