Modality-specific axonal regeneration: toward selective regenerative neural interfaces
- PMID: 22016734
- PMCID: PMC3191531
- DOI: 10.3389/fneng.2011.00011
Modality-specific axonal regeneration: toward selective regenerative neural interfaces
Abstract
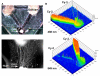
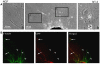
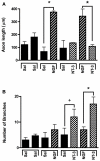
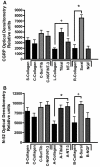
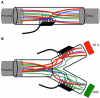
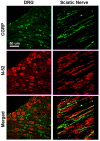
Regenerative peripheral nerve interfaces have been proposed as viable alternatives for the natural control of robotic prosthetic devices. However, sensory and motor axons at the neural interface are of mixed sub-modality types, which difficult the specific recording from motor axons and the eliciting of precise sensory modalities through selective stimulation. Here we evaluated the possibility of using type specific neurotrophins to preferentially entice the regeneration of defined axonal populations from transected peripheral nerves into separate compartments. Segregation of mixed sensory fibers from dorsal root ganglion neurons was evaluated in vitro by compartmentalized diffusion delivery of nerve growth factor (NGF) and neurotrophin-3 (NT-3), to preferentially entice the growth of TrkA+ nociceptive and TrkC+ proprioceptive subsets of sensory neurons, respectively. The average axon length in the NGF channel increased 2.5-fold compared to that in saline or NT-3, whereas the number of branches increased threefold in the NT-3 channels. These results were confirmed using a 3D "Y"-shaped in vitro assay showing that the arm containing NGF was able to entice a fivefold increase in axonal length of unbranched fibers. To address if such segregation can be enticed in vivo, a "Y"-shaped tubing was used to allow regeneration of the transected adult rat sciatic nerve into separate compartments filled with either NFG or NT-3. A significant increase in the number of CGRP+ pain fibers were attracted toward the sural nerve, while N-52+ large-diameter axons were observed in the tibial and NT-3 compartments. This study demonstrates the guided enrichment of sensory axons in specific regenerative chambers, and supports the notion that neurotrophic factors can be used to segregate sensory and perhaps motor axons in separate peripheral interfaces.
Keywords: NGF; NT-3; bionics; multielectrode array; nerve regeneration; neurotrophins; peripheral nerve; sensory feedback.
Figures



References
-
- Benvenuto A., Raspopovic S., Hoffmann K. P., Carpaneto J., Cavallo G., Di Pino G., Guglielmelli E., Rossini L., Rossini P. M., Tombini M., Micera S. (2010). Intrafascicular thin-film multichannel electrodes for sensory feedback: evidences on a human amputee. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2010, 1800–1803 - PubMed
-
- Biran R., Martin D. C., Tresco P. A. (2007). The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. A 82, 169–178 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

