Non-Enzymatic Functions of Retroviral Integrase: The Next Target for Novel Anti-HIV Drug Development
- PMID: 22016749
- PMCID: PMC3192317
- DOI: 10.3389/fmicb.2011.00210
Non-Enzymatic Functions of Retroviral Integrase: The Next Target for Novel Anti-HIV Drug Development
Abstract
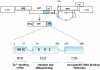
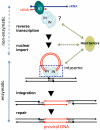
Integrase (IN) is a retroviral enzyme that catalyzes the insertion of viral DNA (vDNA) into host chromosomal DNA, which is necessary for efficient viral replication. The crystal structure of prototype foamy virus IN bound to cognate vDNA ends, a complex referred to as the intasome, has recently been resolved. Structure analysis of the intasome revealed a tetramer structure of IN that was required for its catalytic function, and also showed the inhibitory mechanism of the IN inhibitor. Genetic analysis of IN has revealed additional non-enzymatic roles during viral replication cycles at several steps other than integration. However, the higher order structure of IN that is required for its non-enzymatic functions remains to be delineated. This is the next major challenge in the field of IN structural biology hoping to be a platform for the development of novel IN inhibitors to treat human immunodeficiency virus type 1 infectious disease.
Keywords: Gemin2; HIV-1; intasome; integrase; pol; reverse transcriptase; reverse transcription.
Figures
References
-
- Burke C. J., Sanyal G., Bruner M. W., Ryan J. A., Lafemina R. L., Robbins H. L., Zeft A. S., Middaugh C. R., Cordingley M. G. (1992). Structural implications of spectroscopic characterization of a putative zinc finger peptide from HIV-1 integrase. J. Biol. Chem. 267, 9639–9644 - PubMed
LinkOut - more resources
Full Text Sources

