Insights into the molecular mechanisms of the anti-atherogenic actions of flavonoids in normal and obese mice
- PMID: 22016761
- PMCID: PMC3189911
- DOI: 10.1371/journal.pone.0024634
Insights into the molecular mechanisms of the anti-atherogenic actions of flavonoids in normal and obese mice
Abstract
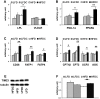
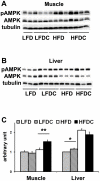
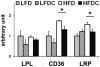
Obesity is a major and independent risk factor for cardiovascular disease and it is strongly associated with the development of dyslipidemia, insulin resistance and type 2 diabetes. Flavonoids, a diverse group of polyphenol compounds of plant origin widely distributed in human diet, have been reported to have numerous health benefits, although the mechanisms underlying these effects have remained obscure. We analyzed the effects of chronic dietary supplementation with flavonoids extracted from cranberry (FLS) in normal and obese C57/BL6 mice compared to mice maintained on the same diets lacking FLS. Obese mice supplemented with flavonoids showed an amelioration of insulin resistance and plasma lipid profile, and a reduction of visceral fat mass. We provide evidence that the adiponectin-AMPK pathway is the main mediator of the improvement of these metabolic disorders. In contrast, the reduced plasma atherogenic cholesterol observed in normal mice under FLS seems to be due to a downregulation of the hepatic cholesterol synthesis pathway. Overall, we demonstrate for the first time that the molecular mechanisms underlying the beneficial effects of flavonoids are determined by the metabolic state.
Conflict of interest statement
Figures




References
-
- Barnes S, Prasain J. Current progress in the use of traditional medicines and nutraceuticals. Curr Opin Plant Biol. 2005;8:324–328. - PubMed
-
- Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S–520S. - PubMed
-
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. - PubMed
-
- Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49:741–781. - PubMed
-
- Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

