Apolipoprotein J/clusterin in human erythrocytes is involved in the molecular process of defected material disposal during vesiculation
- PMID: 22016805
- PMCID: PMC3189238
- DOI: 10.1371/journal.pone.0026033
Apolipoprotein J/clusterin in human erythrocytes is involved in the molecular process of defected material disposal during vesiculation
Abstract
Background: We have showed that secretory Apolipoprotein J/Clusterin (sCLU) is down-regulated in senescent, stressed or diseased red blood cells (RBCs). It was hypothesized that sCLU loss relates to RBCs vesiculation, a mechanism that removes erythrocyte membrane patches containing defective or potentially harmful components.
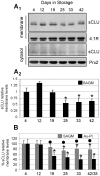
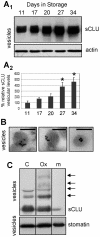
Methodology/principal findings: To investigate this issue we employed a combination of biochemical and microscopical approaches in freshly prepared RBCs or RBCs stored under standard blood bank conditions, an in vitro model system of cellular aging. We found that sCLU is effectively exocytosed in vivo during membrane vesiculation of freshly prepared RBCs. In support, the RBCs' sCLU content was progressively reduced during RBCs ex vivo maturation and senescence under cold storage due to its selective exocytosis in membrane vesicles. A range of typical vesicular components, also involved in RBCs senescence, like Band 3, CD59, hemoglobin and carbonylated membrane proteins were found to physically interact with sCLU.
Conclusions/significance: The maturation of RBCs is associated with a progressive loss of sCLU. We propose that sCLU is functionally involved in the disposal of oxidized/defected material through RBCs vesiculation. This process most probably takes place through sCLU interaction with RBCs membrane proteins that are implicit vesicular components. Therefore, sCLU represents a pro-survival factor acting for the postponement of the untimely clearance of RBCs.
Conflict of interest statement
Figures


References
-
- Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res. 2006;40:1324–1334. - PubMed
-
- Trougakos IP, Gonos ES. Chapter 9: Oxidative stress in malignant progression: The role of Clusterin, a sensitive cellular biosensor of free radicals. Adv Cancer Res. 2009;104:171–210. - PubMed
-
- Bartl MM, Luckenbach T, Bergner O, Ullrich O, Koch-Brandt C. Multiple receptors mediate apoJ-dependent clearance of cellular debris into nonprofessional phagocytes. Exp Cell Res. 2001;271:130–141. - PubMed
-
- Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP-independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding-competent state. Biochemistry. 2000;39:15953–15960. - PubMed
-
- Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, et al. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

