Mesenchymal stem cells restore frataxin expression and increase hydrogen peroxide scavenging enzymes in Friedreich ataxia fibroblasts
- PMID: 22016819
- PMCID: PMC3189234
- DOI: 10.1371/journal.pone.0026098
Mesenchymal stem cells restore frataxin expression and increase hydrogen peroxide scavenging enzymes in Friedreich ataxia fibroblasts
Abstract
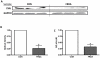
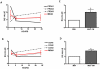
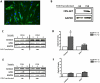
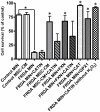
Dramatic advances in recent decades in understanding the genetics of Friedreich ataxia (FRDA)--a GAA triplet expansion causing greatly reduced expression of the mitochondrial protein frataxin--have thus far yielded no therapeutic dividend, since there remain no effective treatments that prevent or even slow the inevitable progressive disability in affected individuals. Clinical interventions that restore frataxin expression are attractive therapeutic approaches, as, in theory, it may be possible to re-establish normal function in frataxin deficient cells if frataxin levels are increased above a specific threshold. With this in mind several drugs and cytokines have been tested for their ability to increase frataxin levels. Cell transplantation strategies may provide an alternative approach to this therapeutic aim, and may also offer more widespread cellular protective roles in FRDA. Here we show a direct link between frataxin expression in fibroblasts derived from FRDA patients with both decreased expression of hydrogen peroxide scavenging enzymes and increased sensitivity to hydrogen peroxide-mediated toxicity. We demonstrate that normal human mesenchymal stem cells (MSCs) induce both an increase in frataxin gene and protein expression in FRDA fibroblasts via secretion of soluble factors. Finally, we show that exposure to factors produced by human MSCs increases resistance to hydrogen peroxide-mediated toxicity in FRDA fibroblasts through, at least in part, restoring the expression of the hydrogen peroxide scavenging enzymes catalase and glutathione peroxidase 1. These findings suggest, for the first time, that stem cells may increase frataxin levels in FRDA and transplantation of MSCs may offer an effective treatment for these patients.
Conflict of interest statement
Figures


Similar articles
-
Mesenchymal Stem Cell-Derived Factors Restore Function to Human Frataxin-Deficient Cells.Cerebellum. 2017 Aug;16(4):840-851. doi: 10.1007/s12311-017-0860-y. Cerebellum. 2017. PMID: 28456899 Free PMC article.
-
Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich's ataxia.Cerebellum. 2012 Dec;11(4):861-71. doi: 10.1007/s12311-012-0406-2. Cerebellum. 2012. PMID: 22826109
-
The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis.Hum Mol Genet. 1999 Mar;8(3):425-30. doi: 10.1093/hmg/8.3.425. Hum Mol Genet. 1999. PMID: 9949201
-
Friedreich ataxia: an update on animal models, frataxin function and therapies.Adv Exp Med Biol. 2009;652:247-61. doi: 10.1007/978-90-481-2813-6_17. Adv Exp Med Biol. 2009. PMID: 20225031 Review.
-
Beyond loss of frataxin: the complex molecular pathology of Friedreich ataxia.Discov Med. 2014 Jan;17(91):25-35. Discov Med. 2014. PMID: 24411698 Review.
Cited by
-
Treatment of spinal cord injury with mesenchymal stem cells.Cell Biosci. 2020 Sep 22;10:112. doi: 10.1186/s13578-020-00475-3. eCollection 2020. Cell Biosci. 2020. PMID: 32983406 Free PMC article. Review.
-
Current Concepts of Stem Cell Therapy for Chronic Spinal Cord Injury.Int J Mol Sci. 2021 Jul 11;22(14):7435. doi: 10.3390/ijms22147435. Int J Mol Sci. 2021. PMID: 34299053 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Factors Restore Function to Human Frataxin-Deficient Cells.Cerebellum. 2017 Aug;16(4):840-851. doi: 10.1007/s12311-017-0860-y. Cerebellum. 2017. PMID: 28456899 Free PMC article.
-
Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich's ataxia.Cerebellum. 2012 Dec;11(4):861-71. doi: 10.1007/s12311-012-0406-2. Cerebellum. 2012. PMID: 22826109
-
Pericytes Extend Survival of ALS SOD1 Mice and Induce the Expression of Antioxidant Enzymes in the Murine Model and in IPSCs Derived Neuronal Cells from an ALS Patient.Stem Cell Rev Rep. 2017 Oct;13(5):686-698. doi: 10.1007/s12015-017-9752-2. Stem Cell Rev Rep. 2017. PMID: 28710685
References
-
- Durr A, Cossee M, Agid Y, Campuzano V, Mignard C, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996;335:1169–1175. - PubMed
-
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. - PubMed
-
- Gakh O, Park S, Liu G, Macomber L, Imlay JA, et al. Mitochondrial iron detoxification is a primary function of frataxin that limits oxidative damage and preserves cell longevity. Hum Mol Genet. 2006;15:467–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

