Combined transcriptomic-(1)H NMR metabonomic study reveals that monoethylhexyl phthalate stimulates adipogenesis and glyceroneogenesis in human adipocytes
- PMID: 22017230
- PMCID: PMC3229183
- DOI: 10.1021/pr200765v
Combined transcriptomic-(1)H NMR metabonomic study reveals that monoethylhexyl phthalate stimulates adipogenesis and glyceroneogenesis in human adipocytes
Abstract
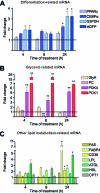
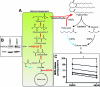
Adipose tissue is a major storage site for lipophilic environmental contaminants. The environmental metabolic disruptor hypothesis postulates that some pollutants can promote obesity or metabolic disorders by activating nuclear receptors involved in the control of energetic homeostasis. In this context, monoethylhexyl phthalate (MEHP) is of particular concern since it was shown to activate the peroxisome proliferator-activated receptor γ (PPARγ) in 3T3-L1 murine preadipocytes. In the present work, we used an untargeted, combined transcriptomic-(1)H NMR-based metabonomic approach to describe the overall effect of MEHP on primary cultures of human subcutaneous adipocytes differentiated in vitro. MEHP stimulated rapidly and selectively the expression of genes involved in glyceroneogenesis, enhanced the expression of the cytosolic phosphoenolpyruvate carboxykinase, and reduced fatty acid release. These results demonstrate that MEHP increased glyceroneogenesis and fatty acid reesterification in human adipocytes. A longer treatment with MEHP induced the expression of genes involved in triglycerides uptake, synthesis, and storage; decreased intracellular lactate, glutamine, and other amino acids; increased aspartate and NAD, and resulted in a global increase in triglycerides. Altogether, these results indicate that MEHP promoted the differentiation of human preadipocytes to adipocytes. These mechanisms might contribute to the suspected obesogenic effect of MEHP.
Figures



Similar articles
-
The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice.Biosci Rep. 2012 Dec;32(6):619-29. doi: 10.1042/BSR20120042. Biosci Rep. 2012. PMID: 22953781 Free PMC article.
-
The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis.J Biol Chem. 2007 Jun 29;282(26):19152-66. doi: 10.1074/jbc.M702724200. Epub 2007 Apr 27. J Biol Chem. 2007. PMID: 17468099
-
Comparative microarray analyses of mono(2-ethylhexyl)phthalate impacts on fat cell bioenergetics and adipokine network.Cell Biol Toxicol. 2017 Dec;33(6):511-526. doi: 10.1007/s10565-016-9380-7. Epub 2017 Jan 12. Cell Biol Toxicol. 2017. PMID: 28083810
-
Activation of PPARalpha and PPARgamma by environmental phthalate monoesters.Toxicol Sci. 2003 Aug;74(2):297-308. doi: 10.1093/toxsci/kfg145. Epub 2003 Jun 12. Toxicol Sci. 2003. PMID: 12805656
-
Proposed involvement of adipocyte glyceroneogenesis and phosphoenolpyruvate carboxykinase in the metabolic syndrome.Biochimie. 2005 Jan;87(1):27-32. doi: 10.1016/j.biochi.2004.12.005. Biochimie. 2005. PMID: 15733733 Review.
Cited by
-
In utero growth restriction and catch-up adipogenesis after developmental di (2-ethylhexyl) phthalate exposure cause glucose intolerance in adult male rats following a high-fat dietary challenge.J Nutr Biochem. 2015 Nov;26(11):1208-20. doi: 10.1016/j.jnutbio.2015.05.012. Epub 2015 Jun 20. J Nutr Biochem. 2015. PMID: 26188368 Free PMC article.
-
Chemical and non-chemical stressors affecting childhood obesity: a systematic scoping review.J Expo Sci Environ Epidemiol. 2018 Jan;28(1):1-12. doi: 10.1038/jes.2017.18. Epub 2017 Sep 27. J Expo Sci Environ Epidemiol. 2018. PMID: 28952603 Free PMC article.
-
Cellular and molecular effect of MEHP Involving LXRα in human fetal testis and ovary.PLoS One. 2012;7(10):e48266. doi: 10.1371/journal.pone.0048266. Epub 2012 Oct 30. PLoS One. 2012. PMID: 23118965 Free PMC article.
-
Monoethylhexyl Phthalate Elicits an Inflammatory Response in Adipocytes Characterized by Alterations in Lipid and Cytokine Pathways.Environ Health Perspect. 2017 Apr;125(4):615-622. doi: 10.1289/EHP464. Epub 2016 Jul 6. Environ Health Perspect. 2017. PMID: 27384973 Free PMC article.
-
Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps.Trends Endocrinol Metab. 2018 Sep;29(9):607-625. doi: 10.1016/j.tem.2018.06.003. Epub 2018 Jul 13. Trends Endocrinol Metab. 2018. PMID: 30017741 Free PMC article. Review.
References
-
- Mullerova D.; Kopecky J. White adipose tissue: storage and effector site for environmental pollutants. Physiol. Res. 2007, 56 (4), 375–381. - PubMed
-
- Grun F.; Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147 (6 Suppl), S50–S55. - PubMed
-
- Desvergne B.; Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999, 20 (5), 649–688. - PubMed
-
- Huber W. W.; Grasl-Kraupp B.; Schulte-Hermann R. Hepatocarcinogenic potential of di(2-ethylhexyl)phthalate in rodents and its implications on human risk. Crit. Rev. Toxicol. 1996, 26 (4), 365–481. - PubMed
-
- Hurst C. H.; Waxman D. J. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003, 74 (2), 297–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

